Stabilities of alkenes can be measured by comparing heats of hydrogenation.
Reaction: C=C + H2 → CH-CH
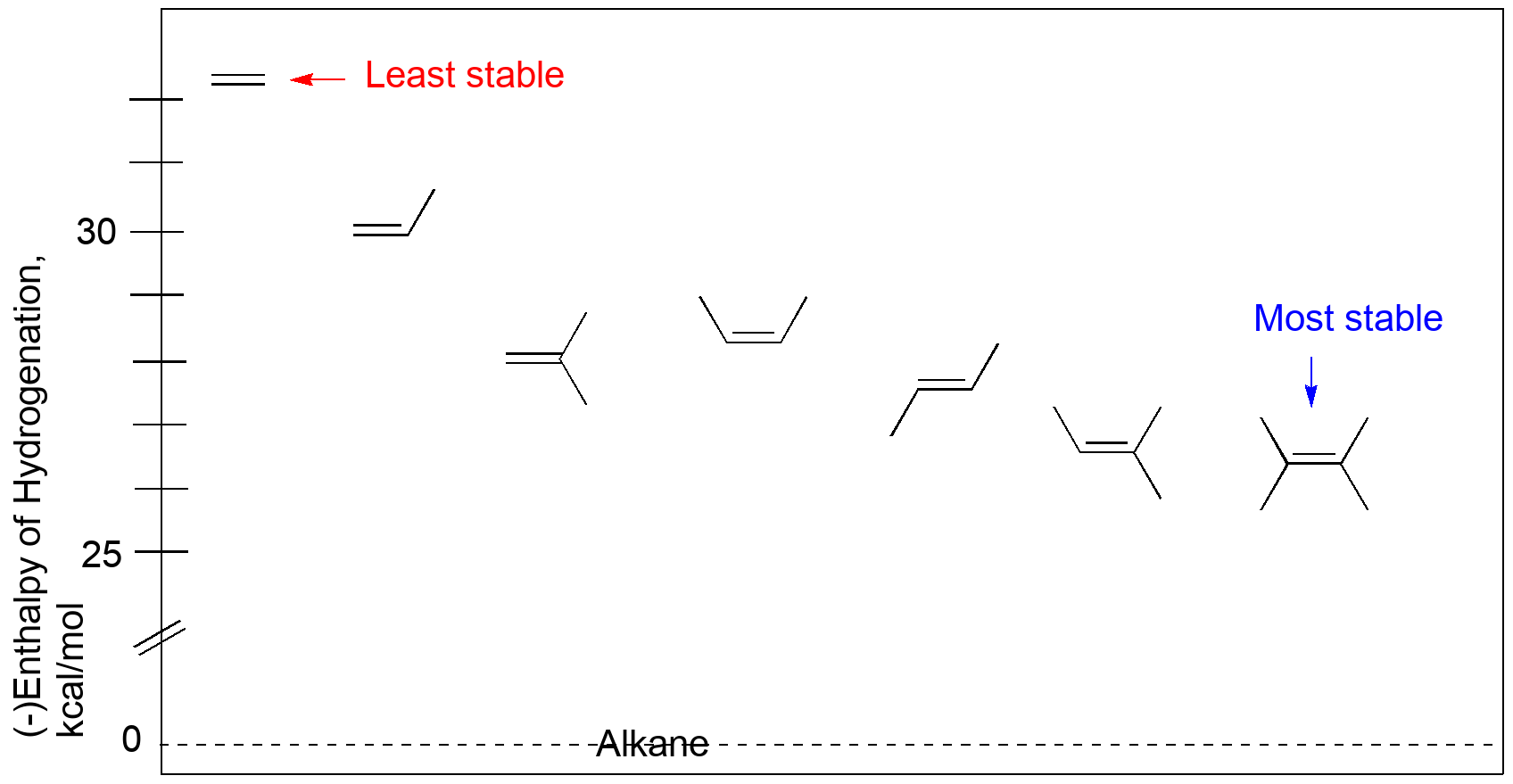
Values (in kcal/mol) taken from the NIST Webbook and reflect more current values. To get values in kJ/mol, multiply by 4.19 J/cal.
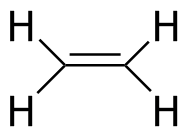
Ethylene |
Monosubstituted |
Average ΔH°r: -30 |
|
 |
-32.4 |
Propene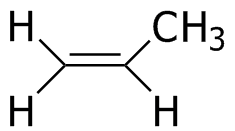 |
-29.5 |
1-Butene |
-30.0 |
||
1-pentene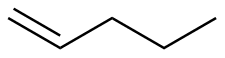 |
-30.0 |
||
1-hexene |
-29.8 |
||
3-methyl-1-butene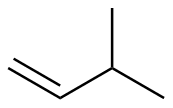 |
-30.1 |
||
3,3-dimethyl-1-butene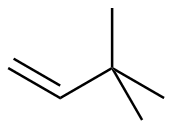 |
-30.0 |
||
E-disubstituted |
Average: -27.4 |
Z-disubstituted |
Average: -28.2 |
E-2-butene |
-27.4 |
Z-2-butene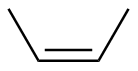 |
-28.3 |
E-2-pentene |
-27,2 |
Z-2-pentene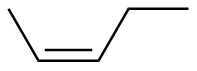 |
-28.1 |
E-2-hexene |
-27.7 |
Z-2-hexene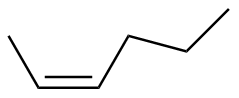 |
-28.5 |
E-3-hexene |
-28.1 |
Z-3-hexene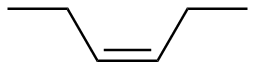 |
-29.0 |
E-4-methyl-2-pentene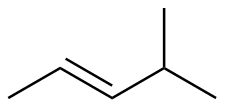 |
-27.3 |
Z-4-methyl-2-pentene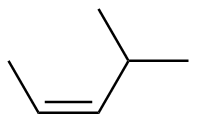 |
-27.9 |
Cyclohexene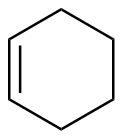 |
-28.3 |
||
Cyclopentene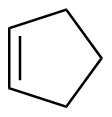 |
-26.9 |
||
Cyclobutene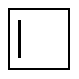 |
-31.5 |
||
1,1-Disubstituted |
Average: -27.9 |
Trisubstituted |
Average: -26.5 |
2-methylpropene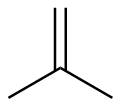 |
-28.1 |
2-methyl-2-butene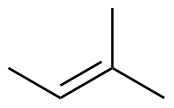 |
-26.6 |
2-methyl-1-butene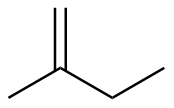 |
-28.2 |
2-methyl-2-pentene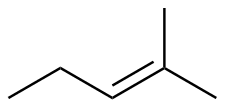 |
-26.6 |
2-methyl-1-pentene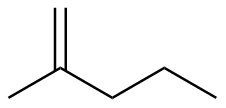 |
-27.8 |
E-3-methyl-2-pentene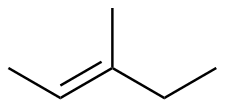 |
-26.3 |
2,3-dimethyl-1-butene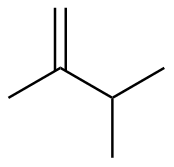 |
-27.8 |
Z-3-methyl-2-pentene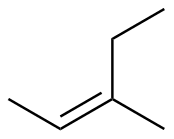 |
-26.4 |
2-ethyl-1-butene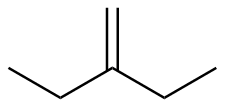 |
-27.6 |
1-methylcyclohexene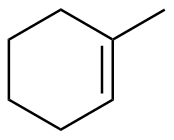 |
-26.6 |
Methylenecyclohexane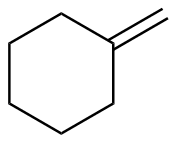 |
-28.5 |
1-methylcyclopentene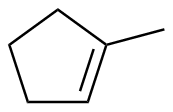 |
-24.0 |
Methylenecyclopentane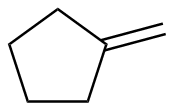 |
-27.7 |
||
Tetrasubstituted |
|||
2,3-dimethyl-2-butene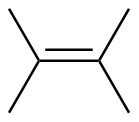 |
-26.3 |