SN1 or SN2?
For each of the following reactions, explain why the mechanism should be SN1 or SN2.
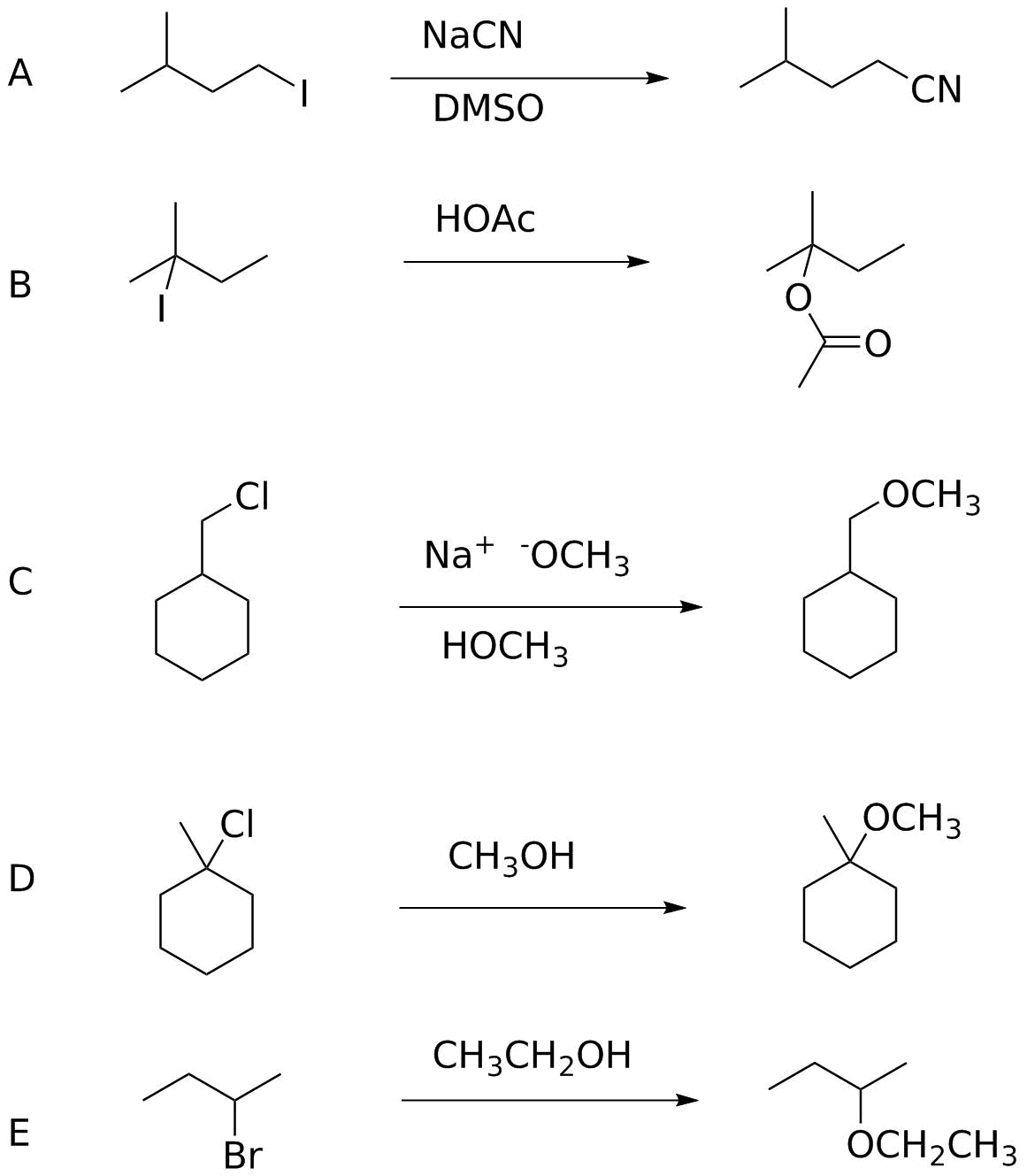
Analysis.
In each of these, evaluate the substrate structure, leaving group ability, nucleophile strength and any impact of solvent on the ability of the system either to form a carbocation (SN1) or achieve backside displacement (SN2).
A. 1° iodide cannot be SN1 (unstable cation); good leaving group, good nucleophile, no unusual solvent effect. SN2.
B. 3° alkyl iodide: good leaving group would form a relatively stable carbocation and the leaving group is good. Cannot undergo SN2 (3°). Weak nucleophile. Everything supports SN1.
C. 1° alkyl chloride, reasonably strong nucloephile (CH3-), polar protic solvent. SN2. (Note that because methoxide is also fairly basic we'd expect some E2 elimination, but the protic solvent would help avoid this.)
D. 3° alkyl chloride, weak but OK leaving group; poor nucleophile. Cannot be SN2 and the relatively stable carbocation expected from C-Cl ionization fits with expecting SN1.
E. 2° substrates can undergo either SN1 or SN2 mechanisms so we have to look elsewhere for clues. We have a good leaving group, a very weak nucleophile and a polar, protic solvent. Those three suggest SN1. Note that a stronger nucleophile and a polar, aprotic solvent would fit better with SN2. (Think about an experiment that you could perform to check. There are two different ways to do that.)
