Substrate reactivity
Which of the following isomeric alkyl bromides would react fastest with NaN3?
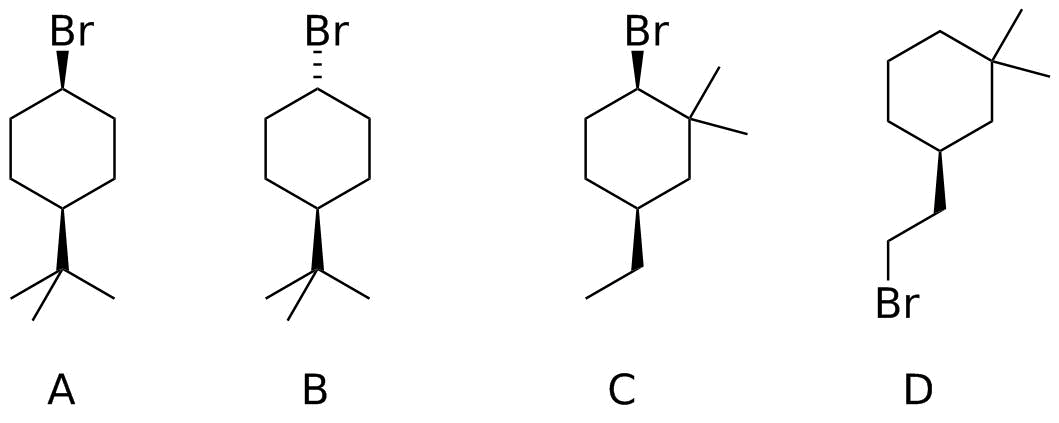
Analysis: The overall reaction is substitution ( SN2 ) of the azide ion N3- for Br-. Azide is a good nucleophile and bromide is a good leaving group; we don't have to worry about adequate reactivity, and of course these pieces are identical in every case.
The big difference is the structure of the organic framework holding the bromide. There are three significant aspects to consider:
- Is the reacting carbon 1°, 2°, or 3°? Only 1° and 2° (as well as methyl) will undergo an SN2 reaction like this. All of the structures are either 1° or 2° bromides. Compound D is 1°, so it should react the fastest.
- Can the nucleophile reach the reacting carbon? The most common feature to worry about is branching adjacent to the reaction center. Compound C is a "neopentyl" halide and because of the branching, it will not react.
- Are there any other factors at play? Comparing compounds A and B, the orientation of the bromine differs. The tert-butyl group is locked into the equatorial position, so in Compound B, the bromide is equatorial also--the nucleophile would have to be inside the ring in order to achieve the right trajectory for backside attack! Compound A, with an axial C-Br bond, can experience attack from the top of the ring. This is slower than for Compound D, but still feasible.
So the order of reactivity is D > A > B = C do not react.
You should build models, but the 3-D structures areshown here in spacefilling models. Compare the accessibility of the back side of each carbon bonded to bromine.
