Identifying problems
Each of the following substitution reactions fails. Describe why. Can any of them be "fixed"?
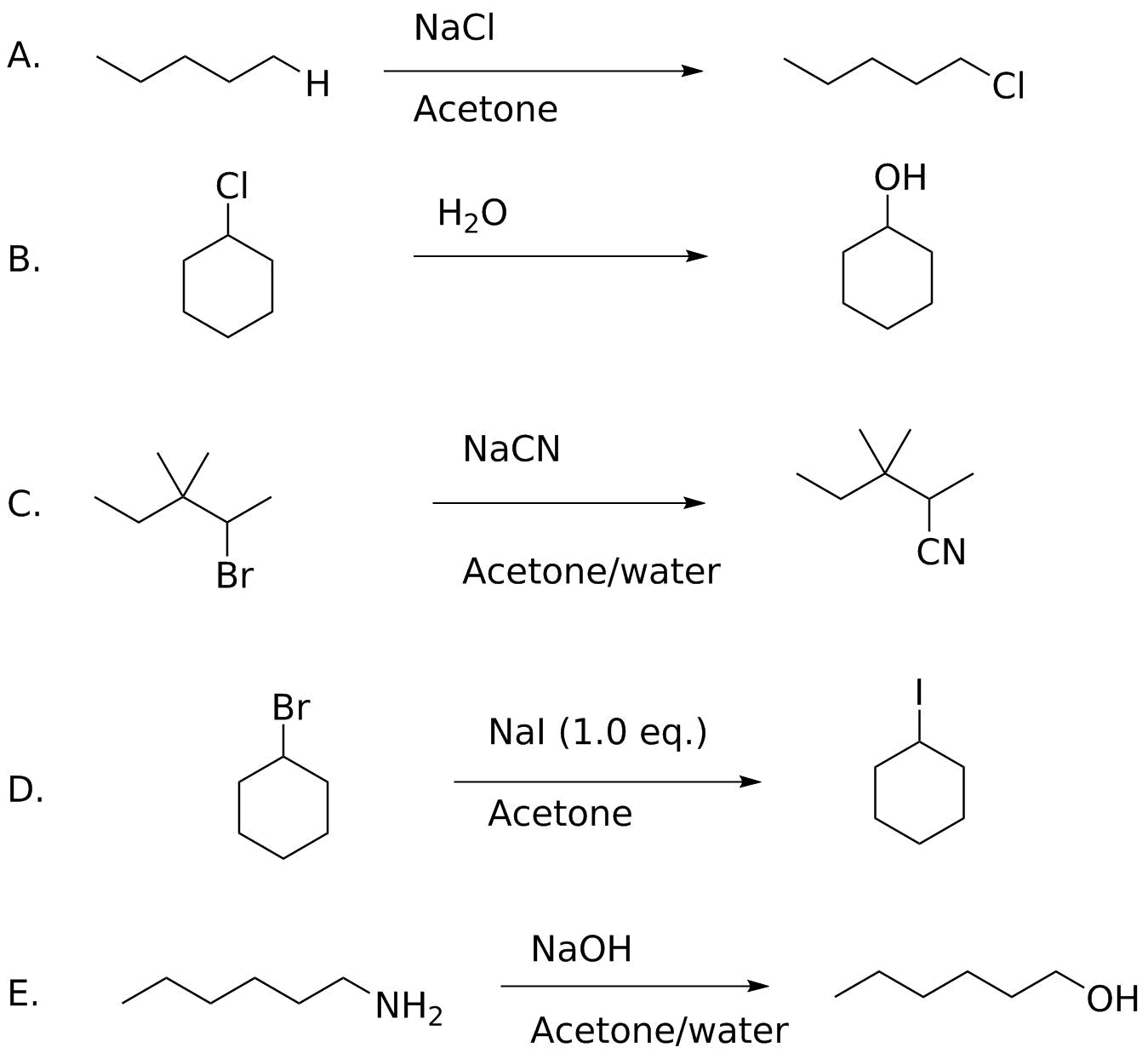
Analysis:
Again, remember the different factors we examined: substrate structure, leaving group ability, nucleophilicity and solvent effects; concentration also plays a role given the rate law for any SN2 reaction. If there are problems with any of these the reaction will fail.
A: This would require H- as a leaving group. Hydride (and carbanions) are NEVER leaving groups. This can't be fixed; free radical halogenation is the only mechanism you have seen that can do this, and the problem with that is selectivity.
B. Water is a poor nucleophile. Hydroxide is a much better nucleophile and so NaOH can accomplish this.
C. The substrate is neopentyl--the adjacent groups on the quaternary carbon prevent the nucleophile from getting in to form the new bond. Unfixable.
D. The C-I bond is weaker than the C-Br bond (56 vs. 70 kcal/mol) so the equilibrium favors the reactant by 14 kcal/mol. In the case of displacement of chloride, recall that precipitation of NaCl drives the reaction, but here both NaI and NaBr are soluble in acetone. The one opportunity would be to increase the [I-] concentration to push the equilibrium to the right.
E. NH2- is a bad leaving group. There are methods to convert it to a better leaving group (diazotization) that you will see in later chapters.
