Comparative Reactivity
For each pair of reactions, select which one would proceed faster.
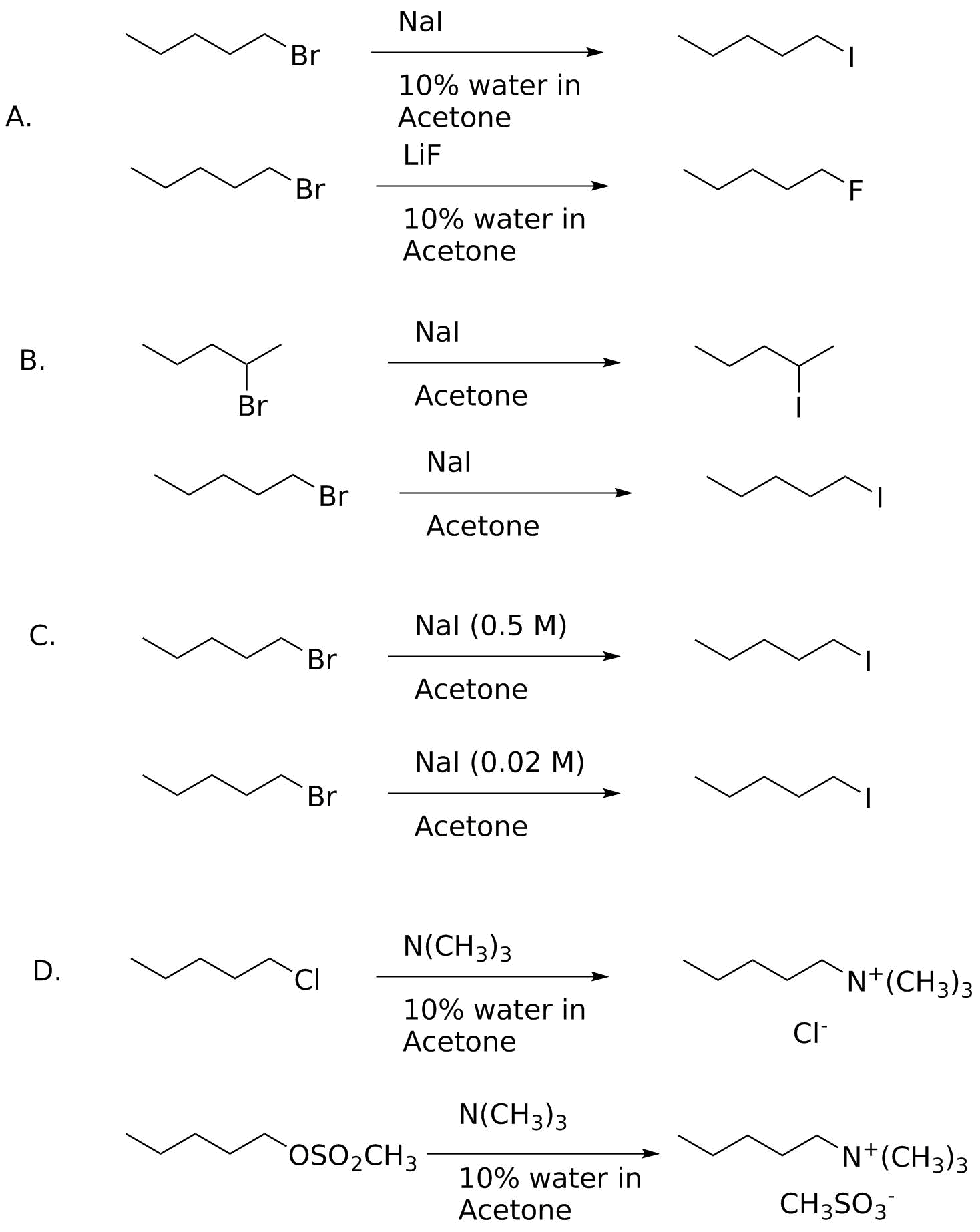
Analysis:
In general, we want to see what is different between the two examples, and decide what factors will favor one versus the other. Remember the different factors we examined: substrate structure, leaving group ability, nucleophilicity and solvent effects; concentration also plays a role given the rate law for any SN2 reaction.
A: Fluoride versus iodide as nucleophile. This is solvent dependent! Aqueous acetone is a protic solvent (the water H-bonds tightly to F-) so iodide will be the stronger nucleophile. (Note that in the absence of water, acetone is polar aprotic, and the reactivities are reversed--although F- then becomes a better base, too.)
B. Substrate stricture; 1° > 2°.
C. Nucleophile concentration; rate = k2[Nu][substrate]. The higher concentration proceeds faster.
D. Leaving groups; methanesulfonate is better than chloride.
