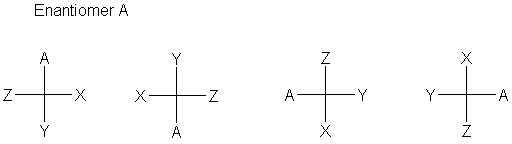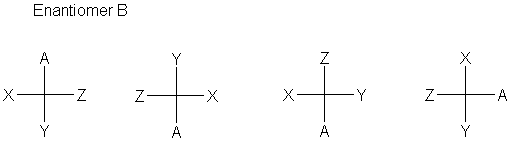Molecules that have multiple stereocenters become difficult to draw quickly. The Fischer projection is a convention for drawing molecules and quickly designating stereochemistry without laboriously trying to use wedges and hashes to specify the 3-D relationships.
A historical note: this was developed at a time before it was possible to experimentally prove absolute configuration for any molecule. Emil Fischer, an early carbohydrate chemist, developed it so that it could continue to be used correctly and consistently even after experimental proof of configuration was developed.
The definition is that every carbon is specified completely by a cross designating the carbon (at the center) and the four bonds to that carbon. The stereochemistry of the bonds is defined (now) as the horizontal bonds are in front of the plane (coming toward you, the viewer); the vertical bonds are behind the plane (going away from you). Sometimes (often, even), bonds to hydrogen are omitted.
The implementation for glyceraldehyde (2,3-dihydroxypropanal) is shown below.
| One configuration of glyceraldehyde | The enantiomer (actually, the naturally-occurring form) | |
|
Label R/S Black background White background |
||
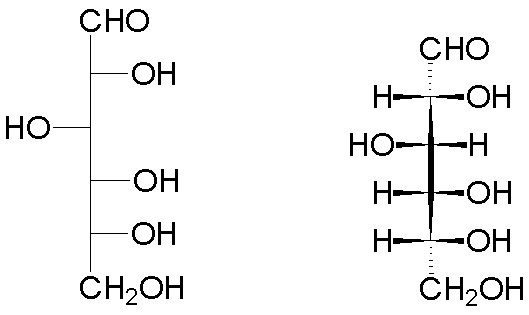 |
The normal convention is to have the main carbon chain run vertically, though this is not at all required by the convention.
There are some regular relationships that are "short cuts" to recognizing stereochemical relationships:
- A 180° rotation gives the same stereochemistry.
- A 90° rotation gives the inverted stereochemistry.
- Exchanging any two positions gives the inverted stereochemistry.
- Pairwise exchange of two sets of positions gives back the same stereochemistry.