Drawing cyclohexane is an important skill: it helps teach you to present a two-dimensional image that can correctly and concisely convey 3-dimensional information.
First the ring. The major conformer for most cyclohexanes is the chair:
To draw this, keep in mind one important rule:
Bonds that are parallel in the real molecule should be drawn parallel in the picture.
Our general goal is to provide an accurate side-on perspective of the ring, so it's useful to make a model and draw bonds in pairs as you see them:
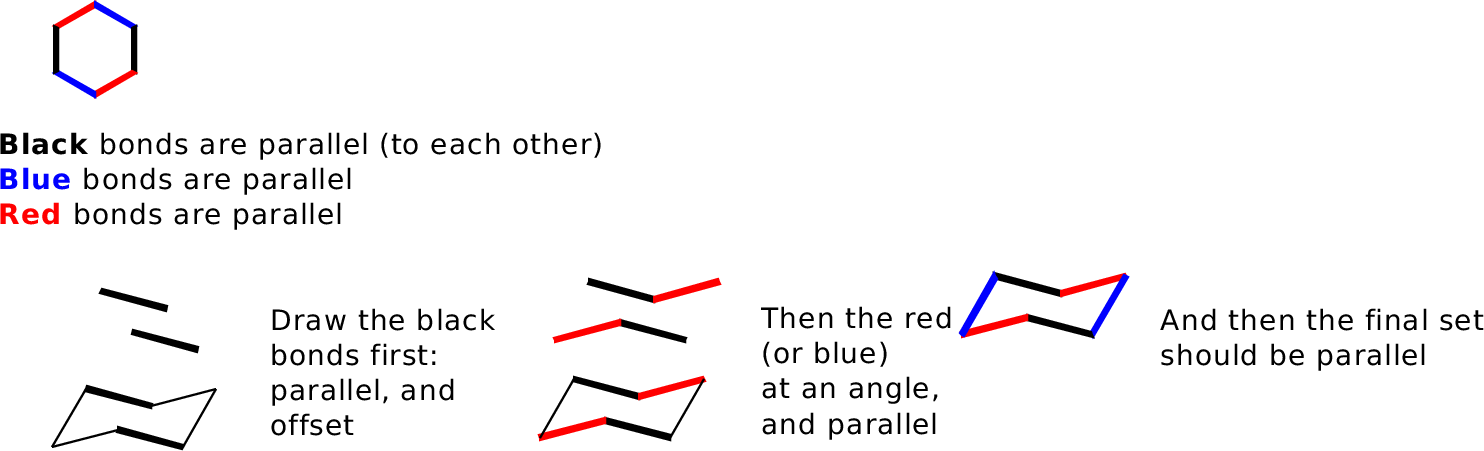
You will need to practice this.
Once you have mastered this, the placement of substituents follws relatively easily.
There are two kinds of substituents: equatorial, in the general plane of the carbons, and axial, oriented perpendicular to this plane.
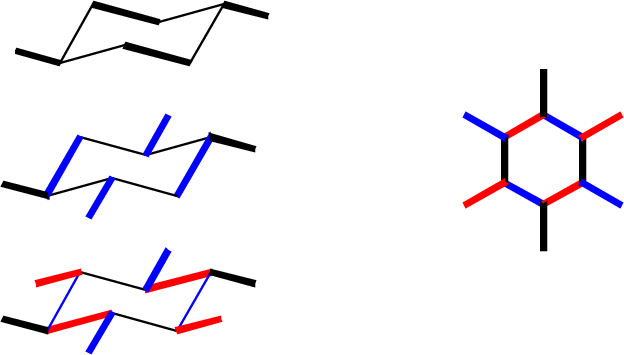
Axial bonds are easiest to draw: they are all vertical, and drawn away from the vertex at each carbon position. There are three "up" and three "down."
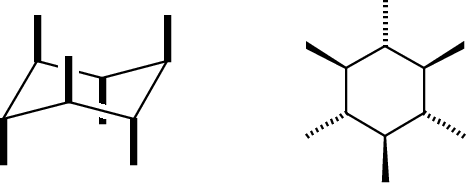
Equatorial bonds need to be drawn parallel to the C-C bonds to which they are parallel in real life:
The chair is the most important form of cyclohexane, but not the only one. Others are the boat (a high-energy form; rarely seen), a half chair (very high energy) and a twist-boat (an intermediate in the ring-flip isomerism between two chair forms). Make models of these and compare their appearance and behavior to Figure 4-9 in the textbook.
The boat is pretty easy to draw, but I do not expect you to master drawing the twist-boati or half-chair. None of these three is a significant component at room temperature.
| Conformer (and energy relative to the chair) See Figure 4.9 (p. 142) in your book to see the reaction energy diagram connecting these in a ring flip. |
Jmol
structure Black background White background Spacefilling model Wireframe Ball & Stick |
Half-chair
(+45.2 kJ/mol, +10.8 kcal/mol)
|
CH_HalfChair.pdb |
Twist-boat
(+23 kJ/mol, +5.5 kcal/mol)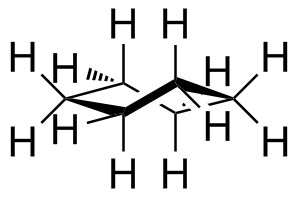
|
cyclohexane_TwBoat.pdb |
Boat (+29
kJ/mol, +6.9
kcal/mol)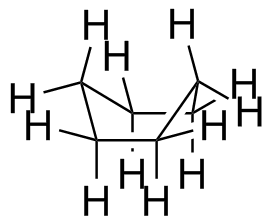
|
cyclohexane_boat.pdb |