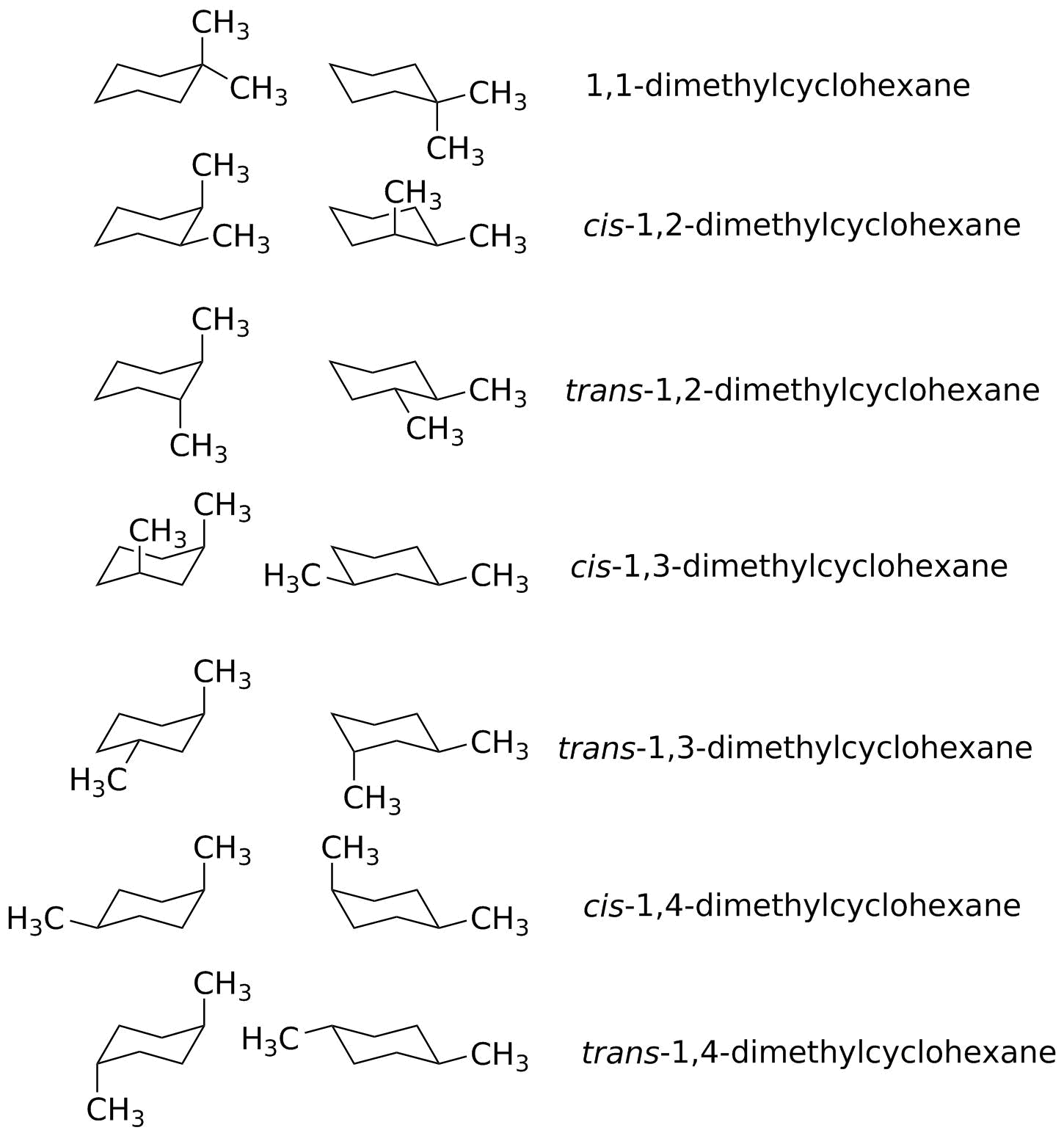
| trans-1,4-dimethylcyclohexane Erel = 0 |
|
| cis-1,3-dimethylcyclohexane Erel = 0 |
|
| trans-1,2-dimethylcyclohexane Erel = +0.56 kcal/mol The gauche effect isn't quite as big as predicted, but still evident. |
|
| 1,1-dimethylcyclohexane Erel = +0.69 kcal/mol This is the oddball since we predicted it to be +1.8 kcal/mol; there is something called the "Thorpe-Ingold effect" that stabilizes geminal dimethyl groups. Discussion of this is beyond the scope of this course. |
|
| trans-1,3-dimethylcyclohexane Erel = +1.69 kcal/mol |
|
| cis-1,4-dimethylcyclohexane Erel = +1.76 kcal/mol |
|
| cis-1,2-dimethylcyclohexane Erel = +2.32 kcal/mol Again, the gauche effect is a little smaller than expected but still evident. |