| |
CH334Organic Chemistry
|
 |
| |
||
| |
||
| |
Drawing cyclohexane is an important skill: it helps teach you to present a two-dimensional image that can correctly and concisely convey 3-dimensional information. First the ring. The major conformer for most cyclohexanes is the chair: To draw this, keep in mind one important rule: Bonds that are parallel in the real molecule should be drawn parallel in the picture. Our general goal is to provide an accurate side-on perspective of the ring, so it's useful to make a model and draw bonds in pairs as you see them: 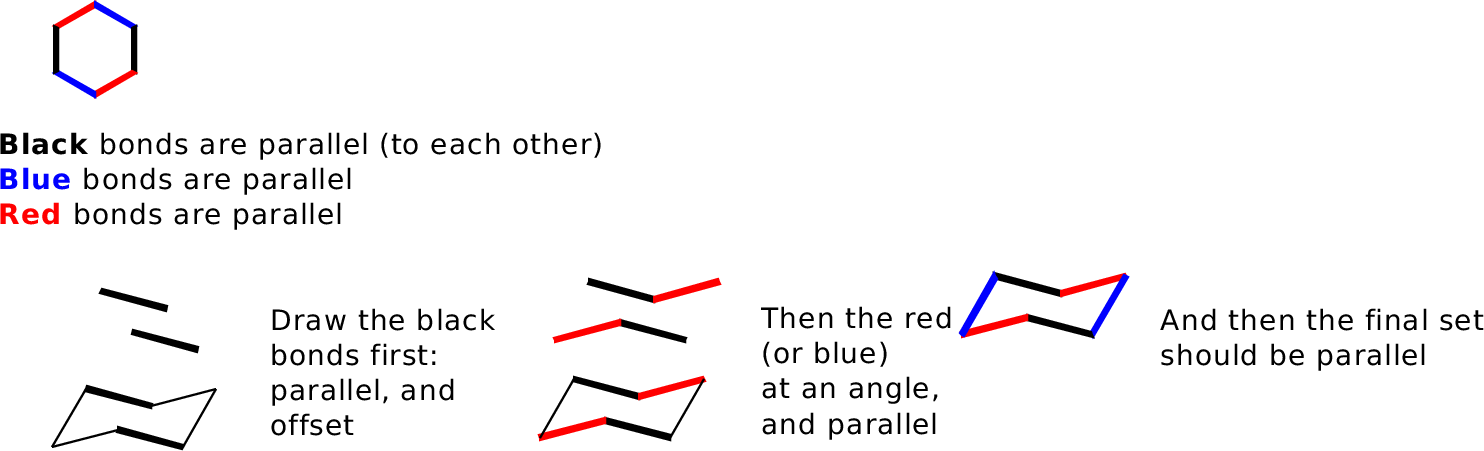 You will need to practice this. Once you have mastered this, the placement of substituents follws relatively easily. There are two kinds of substituents: equatorial, in the general plane of the carbons, and axial, oriented perpendicular to this plane. Axial bonds are easiest to draw: they are all vertical, and drawn away from the vertex at each carbon position. There are three "up" and three "down." 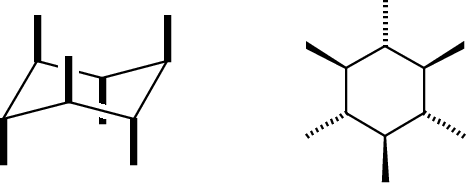 Equatorial bonds need to be drawn parallel to the C-C bonds to which they are parallel in real life: 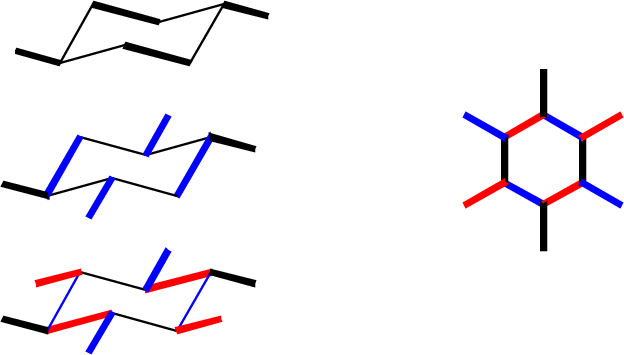 The chair is the most important form of cyclohexane, but not the only one. Others are the boat (a high-energy form; rarely seen) and a twist-boat (an intermediate in the ring-flip isomerism between two chair forms): The boat is pretty easy to draw, but I do not expect you to master drawing the twist-boat. Neither is a significant component at room temperature.
|