Black background
White background
Spacefilling model
Ball & Stick
The net reaction

This is a net substitution of Cl for H in methane, producing HCl as a byproduct.
chlorination_CH4.xyz
Initiation
The first step of chlorination is generation of an odd-electron species. A chlorine molecule absorbs a photon, exciting a bonding electron into an antibonding MO. This destroys the bond and leads to two chlorine atoms.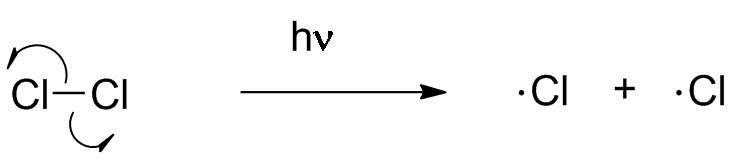
chlorination_CH4.xyz
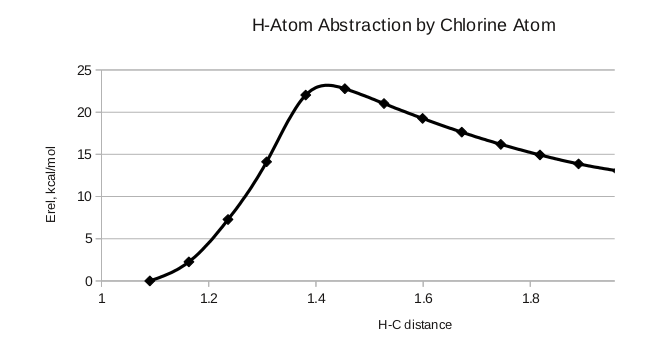
Note the change in hybridization at carbon in the structure above, and the activation barrier for the reaction in the energy plot to the right.
Propagation
Initiation does not lead to any significant net change--only a small portion of chlorine molecules get split into atoms. The production of products involves two steps. In general, radical transformations can involve many steps, but the key principle is that the radical species that starts the cycle is regenrated at some point.The first step occurs when a chlorine atom approaches methane and picks off a hydrogen atom, leaving a methyl radical. This is endothermic by 2 kcal/mol.
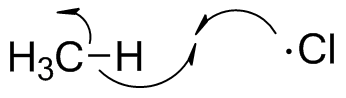
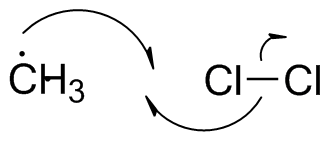
Note the use of the "fishhook" arrows to show electron flow of single electrons.
Ch3_Cl2.xyz
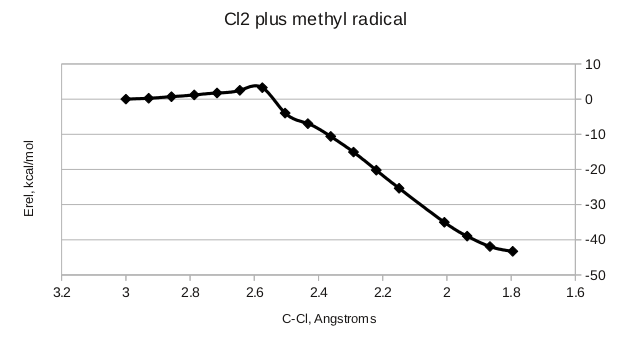
Again, we rehybridize when we form a new bond. There is a smaller activation barrier, indicating that the methyl radical is more reactive than the chlorine radical.
Termination
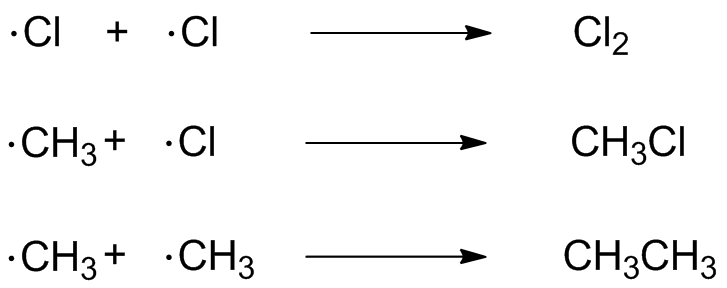
For most of the reaction, the low concentration of radicals means that propagation is the most likely process--a chloring atom is much more likely to find methane than anything else. A methyl radical is much more likely to find Cl2 than anything else. However, over time, radicals start to find each other; radical recombination is a low-barrier, fast reaction, and removes radicals thereby shutting down the propagation cycle.Here, three termination reactions happen: