Synthetic Utility
Select which of the following reactions will be synthetically useful, and explain why.
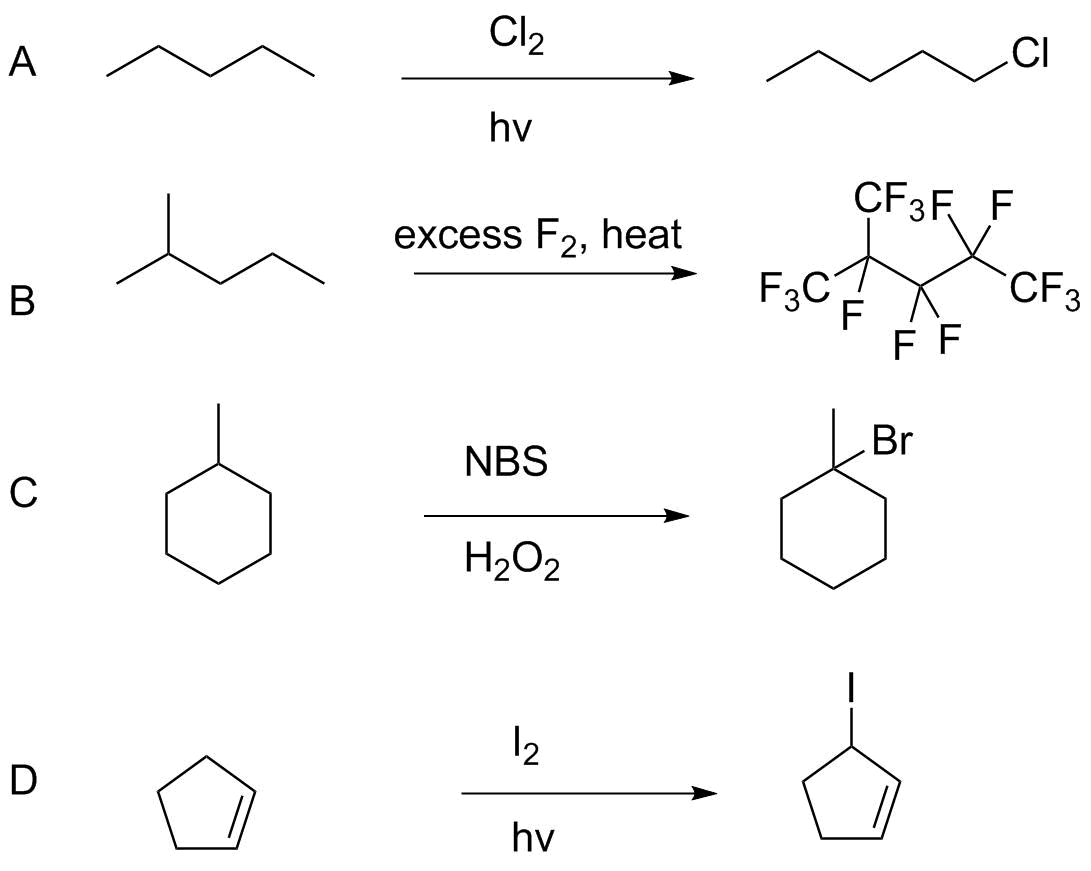
Analysis:
Each one must be examined on the basis of two questions:
- If the overall transformation exothermic, on the basis of BDE values?
- In the H-atom abstraction step, would reaction of the necessary H atom(s) be selective?
The answer must be "yes" for each for the reaction to be synthetically useful. We'll go through each in turn.
A. The overall reaction of a primary C-H (BDE=101 kcal/mol, 423 kJ/mol) with Cl2 (BDE=58 kcal/mol, 243 kJ/mol) to form C-Cl (84 kcal/mol, 352 kJ/mol) and H-Cl (103 kcal/mol, 432 kJ/mol) is exothermic, ΔH° = -28 kcal/mol or -117 kJ/mol. However, abstraction of the primary hydrogen will be nonselective; the secondary hydrogens will react somewhat faster and give as mich or more of the 2-chloro and 3-chloropentane isomers.
B. This is an exhaustive fluorination, so selectivity is not an issue (ignoring whether the introduction of F on a carbon so reduces reactivity of the next hydrogen as to stop substitution--it does not). Using the strongest C-H bond, the overall enthalpy involves cleavage of the primary C-H (BDE=101 kcal/mol, 423 kJ/mol) and F-F (BDE = 37.5 kcal/mol, 157 kJ/mol) to form a C-F bond (111 kcal/mol, 464 kJ/mol) and H-F (136 kcal/mol, 569 kJ/mol). Net ΔH° = -100 kcal/mol or -420 kJ/mol. Substitution of other C-H bonds will be similarly or more favorable. If you are concerned about initiation, the F-F BDE is weak enough to initiate the reaction thermally.
C. NBS and hydrogen peroxide is a standard set of reagents for free radical bromination because NBS generates Br2 under the reaction conditions. The overall reaction of a 3° C-H bond is exothermic; C-H (96.5 kcal/mol/404 kJ/mol), Br-Br (46 kcal/mol/192 kJ/mol) gives C-Br (71 kcal/mol, 297 kJ/mol) and H-Br (87 kcal/mol, 364 kJ/mol). Net ΔH° = -25.5 kcal/mol, -107 kJ/mol. The bromine atom is selective for 3°>2°>1° and so this would be effective.
D. We have a weaker C-H bond at the allylic position--88 kcal/mol (369 kJ/mol). And the I-I bond is very weak; 36 kcal (151 kJ/mol). However the product bonds are even weaker in aggregate: C-I = 55 kcal/mol (230 kJ/mol) and H-I = 71 kcal/mol (297 kJ/mol), so the net reaction is not exothermic: ΔH° is approximately zero. The reaction would not be useful.
