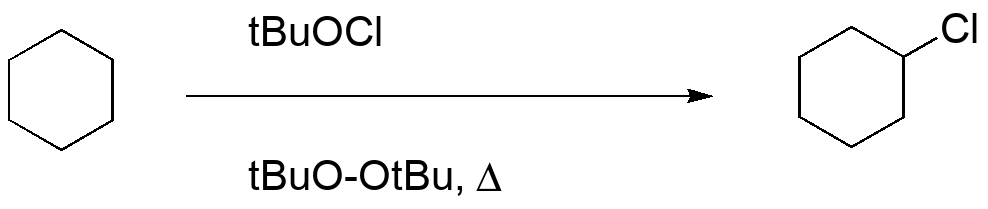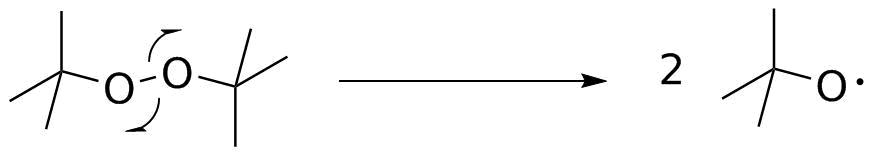Chlorination using Hypochlorites
Draw the mechanism for the initiation and propagation steps for free radical chlorination of cyclohexane using tert-butyl hypochlorite.
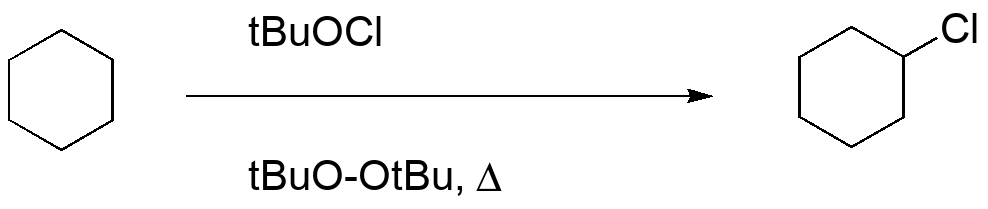
Analysis:
You are told this is a free radical mechanism. We are substituting Cl for H in cyclohexane, so we must find a way to break a C-H bond (most likely by radical H atom abstration) and then have the radical that forms create a new C-Cl bond.
Initiation.
The conditions listed do not include light, but only heat (Δ). There must be a weak bond in one of the reagents that can be easily broken by heating. The O-O bond in di-tert-butylperoxide qualifies (43 kcal/mol), so we can initiate the reaction with a thermal, homolytic bond cleavage:
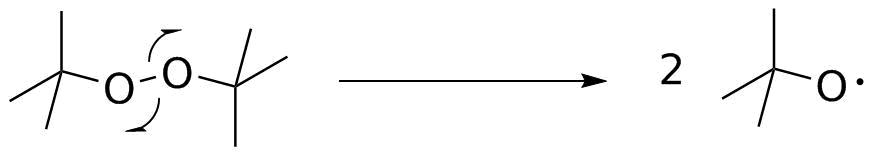
Propagation.
The tert-butyloxy radical now needs to act on cyclohexane, which is present in a large excess. H-atom abstraction requires breaking a C-H bond (secondary, 98 kcal/mol) and forming an O-H bond (120 kcal/mol). This is exothermic (OK so far--but we need to see the rest of the process).
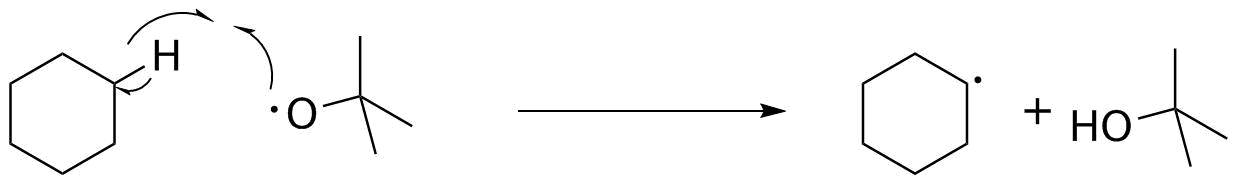
Now, what happens to the cyclohexyl radical? Remember that it is only ever generated in a very small amount at one time. It is not going to find another radical for a long time, but it will bump into tert-butyl hypochlorite. That has a relatively weak (52 kcal/mol) O-Cl bond, and Cl atom abstraction both forms the observed C-Cl bond, and regenerates another tert-butyloxy radical. That cycles back and repeats the process to carry the chain.
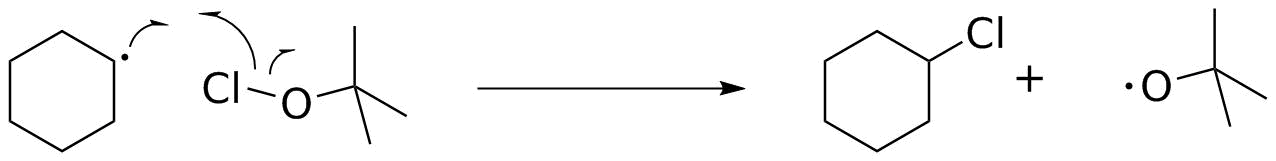
This is also exothermic (C-Cl = 71 kcal/mol) so the overall process is favorable by about 41 kcal/mol (172 kJ/mol). Termination steps will generally be radical recombinations, though there will also be H-atom abstration from the cyclohexyl radical to form a tiny amount of cyclohexene. We are typically not concerned with termination (and the problem does not ask that of you). Interestingly, because this system uses a different radical to break the C-H bond, it's more selective than photochemical chlorination with Cl2, though not as selective as bromination.