Free Radical Bromination
Draw the mechanism for the propagation steps for free radical monobromination of toluene (methylbenzene).
Analysis:
An obvious first step is to draw the reactants, followed by consideration of the possible products. You should recognize that this is a substitution reaction, replacing H with Br. Toluene has 4 distinct types of C-H bond that might be brominated:
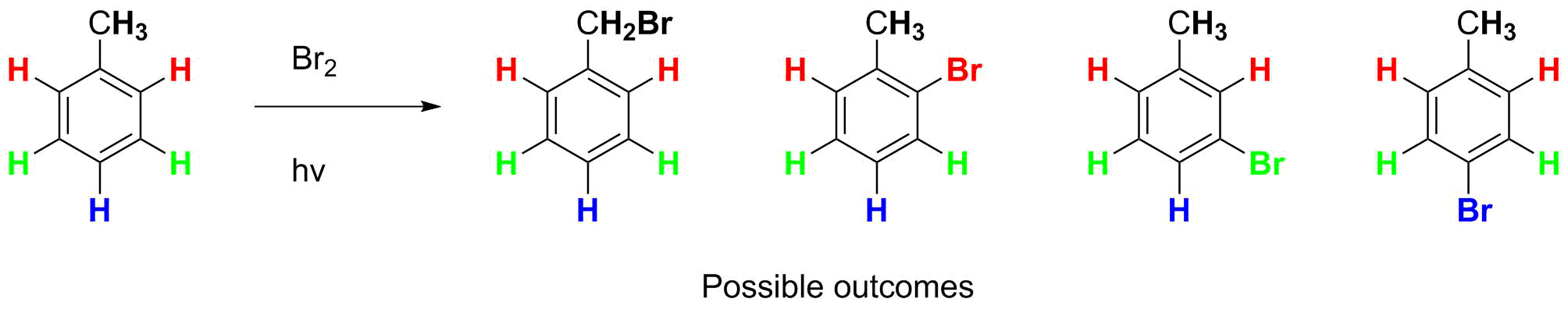
We now have to solve whether there will be any selectivity and if so, why and for what isomer(s). That involves writing out the general mechanism, and then applying it here.
Step 1 What is the species causing the reaction? While not specifically part of the question, you do need to recognize that Br2 + hν generates Br• atoms. However, only a TINY fraction of bromine molecules get split to initiate the reaction.
Step 2 What can this reacting species (Br•) do? The bromine radical really wants to complete its octet and the best way to gain one more electron is by an H-atom abstraction reaction. Bromine is on the low end of the reactivity scale of halogens, and it will selectively break the weakest C-H bond present. The aromatic C-H bonds all have BDEs of about 110 kcal/mol (462 kJ/mol), but the CH2-H bond is weaker. A primary C-H like this in an alkane would have a BDE of 101 kcal/mol (423 kJ/mol), but this is also next to a benzene ring. That stabilizes the radical and weakens the C-H bond--the BDE is only 88 kcal/mol (380 kJ/mol). The H-Br bond that forms is worth 87 kcal/mol (364 kJ/mol) and we get much, though not all, of the energy back. So the CH3 group will react selectively with Br•.
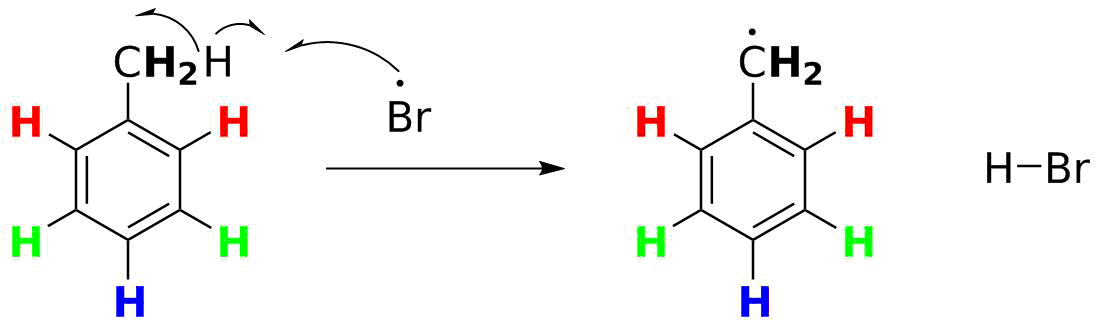
Step 3 What happens to the organic free radical generated here?
This is where many students falter. There is a temptation to simply have the organic radical pair up with a Br• atom to form the desired C-Br bond. This is a "termination" reaction. Remember what we noted in Step 1? only a TINY fraction of Br2 gets converted to Br• atoms. And thus at any one time only a TINY fraction of toluene will be converted to the radical. Let's say the fraction is a millionth (10-6) of the concentration of each. The likelihood of two radicals finding each other is now 1/(1012)--vanishlingly low. The organic radical is a million times more likely to run into a Br2 molecule--and we can apply the same principle as in Step 1:
- The radical wants to complete its octet of electrons.
- It can do so my grabbing an atom from a weak bond, taking an electron with it.
- The Br-Br bond is weak (46 kcal/mol, 192 kJ/mol), and reaction to form the C-Br bond (71 kcal/mol, 197 kJ/mol) is exothermic.
- Breaking the Br-Br bond generates a new Br• atom, which can cycle back and react with another molecule of toluene (thus making a "chain reaction").
So the formal, complete mechanism for the monobromination is:
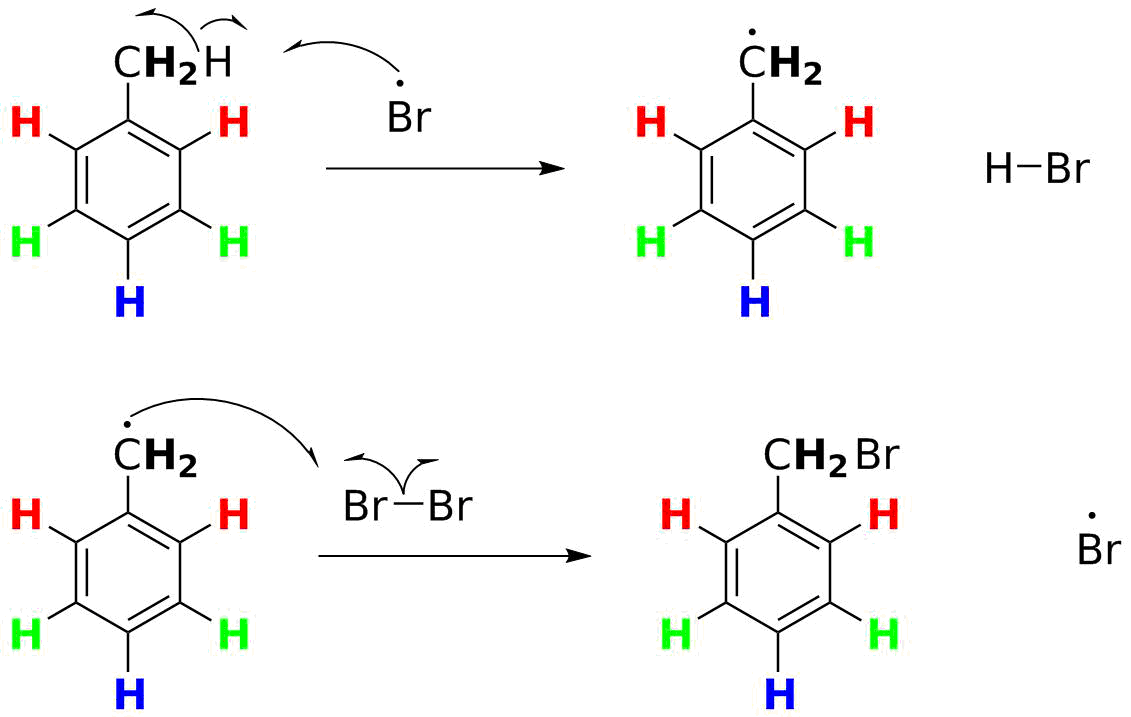
You should be able to do this for ANY alkane and ANY halogen, given a table of BDE values like Table 3-1. You should also be able to calculate the net ΔH° for any given monohalogenation.


