Butane provides a relatively simple demonstration of the energetic issues we face in evaluating rotational isomerism. Our goal is to understand several things:
- What will be the most stable conformer of an alkane (or other molecule?
- What general principles can we find in order to make a prediction for a molecule we've never seen?
- What are the underlying physical laws that control energy vs. conformation?
- How should we think about what a collection of molecules will "look like"?
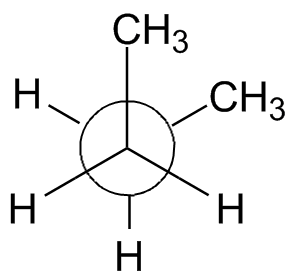
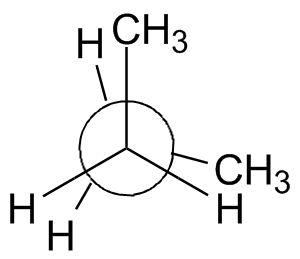
A very useful drawing convention is the Newman projection, named after the chemist who invented it in the 1960s. To draw a Newman projection, use the following process:
- Draw a circle. This represents the bond down which we look.
- If the front carbon is sp3-hybridized, draw three lines that meet at the center of the circle, 120° apart. (An sp2 carbon will use 2 lines 180° apart)
- If the back carbon is sp3-hybridized, draw three lines outside the circle that stop at the circle's edge. These three will be 120° apart; the angular separation between a front-atom bond and a rear-atom bond is the dihedral angle.
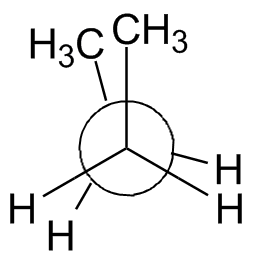
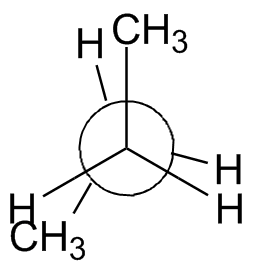
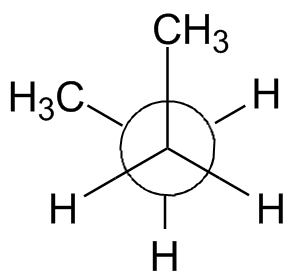
The rotation about the center bond in butane is shown in the chart below using 3-D Jmol structures. You should build a model. Compare to Figure 2-13 in the text and the Newman projections shown.
|
Syn: Butane 0° Gauche: Butane 60° Eclipsed: Butane 120° Anti: Butane 180° Eclipsed: Butane 240° Gauche: Butane 300° Syn: Butane 360° |
|
|
| Newman projection: | 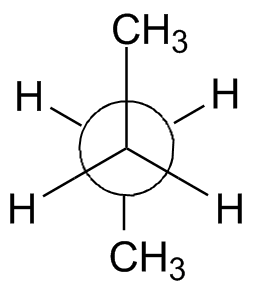
|
|
|

|
|
|

|
| Staggered,
anti, 180° |
Eclipsed,
240° |
Staggered,
gauche, 300° |
Eclipsed,
syn, 360° |
Staggered, gauche,
60° |
Eclipsed,
120° |
Staggered,
anti, 180° |
|
| Energy: 0 |
Energy:
+3.6 kcal/mol (+16 kJ/mol) |
Energy:
+0.9 kcal/mol (+3.8 kJ/mol) |
Energy: +5
kcal/mol (+19 kJ/mol) |
Energy:
+0.9 kcal/mol (+3.8 kJ/mol) |
Energy:
+3.6 kcal/mol (+16 kJ/mol) | ||
- Staggered conformations are more stable than eclipsed
- Anti conformations are most stable; each gauche interaction costs about 0.9 kcal/mol.
The energies tell us how much of any form will be present in a collection of the molecules. Remember the equation for equilibrium:
ΔG° = -RTlnKeq
The equilibrium between anti and gauche is a function of the energy difference (0.9 kcal/mol) and comes out to be Keq = 4.57 at room temperature. This works out to a mixture that is 82% anti, 18% gauche. (Work out the K for the eclipsed conformations, and you will find there will be <0.1% of either.)