Problem: What is the most acidic hydrogen?
Determine the most acidic H atom in the following molecule.
Analysis
First, identify all different types of hydrogen. There are two levels of doing this:
- What atom is the hydrogen connected to? Generally, O-H hydrogens are more acidic than N-H, which in turn are greater than C-H bonds. However...
- Are there other factors (resonance stabilization in particular) that will stabilize the deprotonated form and make the hydrogen more acidic?
- Finally, is there a molecule where you know the pKa that has a comparable hydrogen? Can you estimate the pKa of each hydrogen? (see Table 2.2, or memorized benchmark compounds)
In this case we have many C-H bonds, but the range of pKa values is all 42-50--these are the weakest acids present. (There is one C-H that might be as acidic as pKa=35.) There is one N-H bond (similar to NH3--pKa = 38), and two O-H protons. One of these is an alcohol (similar to H2O--pKa = 15.7), and the other looks like acetic acid--pKa = 4.74. The carboxylic acid proton is thus the most acidic proton by a factor of 1011. The resonance stabilization of the anion is the most significant factor in determining this.
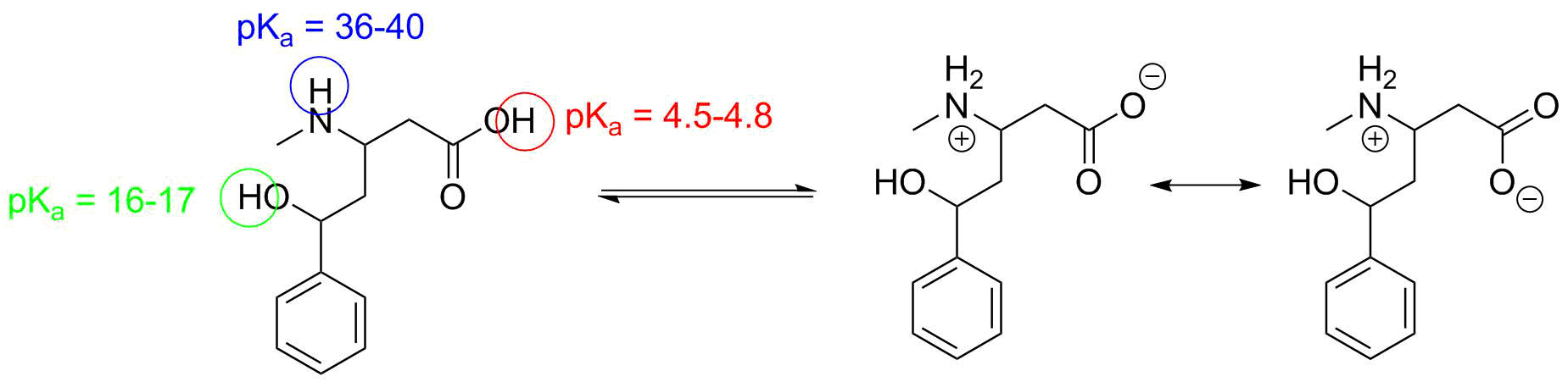
One aspect that is not--formally--part of the question but is worth identifying is that the amine is actually a strong enough base to deprotonate the acid internally (the pKa of the conjugate acid is about 10). So the predominant form of the molecule is a "zwitterion".

