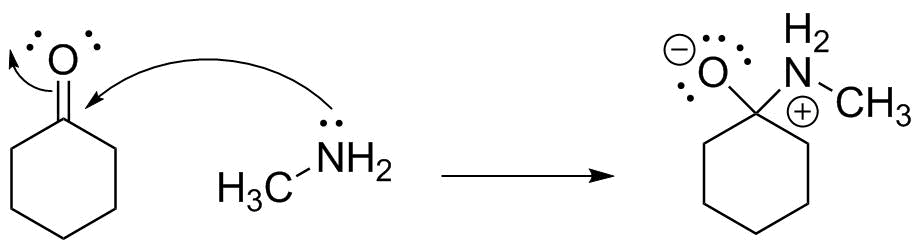Problem: Curved arrows.
Use curved arrows to show the reaction between methylamine (CH3NH2) with cyclohexanone.
Analysis
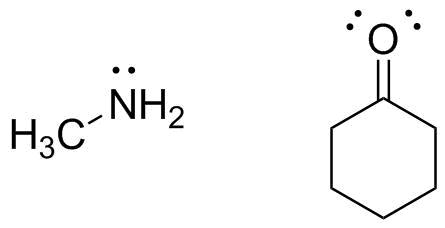
You must initially identify (and draw) the reactants. Because this deals with illustrating electron motion during a reaction, we want to draw complete Lewis structures that show all lone pairs. (If the reaction were to involve a C-H bond, you would walt to explicitly show the bond line for that C-H bond.)
The next step is to identify the nucleophile (the Lewis base--the source of electrons) and the electrophile (the Lewis acid--the electron acceptor). This requires you to look at EACH reactant and determine whether it has Lewis acidic or basic atoms, and if there are multiple possibilities, which is most effective in either role. You may (and in this case must) have to consider alternative resonance structures to identify this reactivity.
The methylamine is straightforward. There are no electron deficient atoms, but the nitrogen has a lone pair, making it Lewis basic and a nucleophile. This lone pair will be donated to make a new bond.
The cyclohexanone is more of a challenge. The oxygen lone pairs make it a Lewis base, but there would then be no lone pair acceptor. All other atoms have a complete octet as drawn, so identifying a Lewis acid might be a challenge--until we remember to look at resonance:
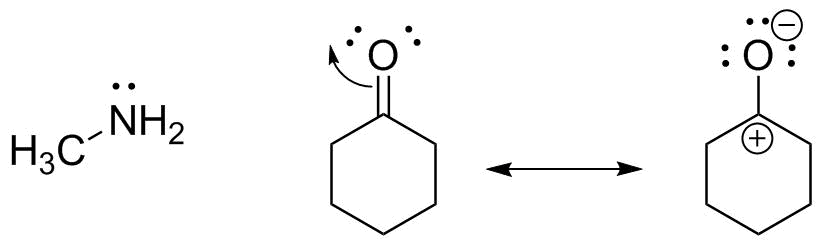
This second resonance form shows that the C=O carbon has electron deficient character and can act as a Lewis acid to accept an electron pair.
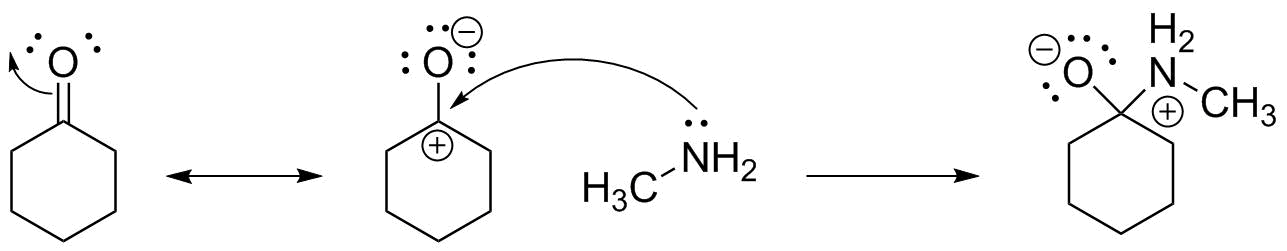
Now that we recognize this, we know that the bond will form between the nitrogen (lone pair donor) and the carbon (lone pair acceptor). There are two acceptable ways to draw this: we can write the resonance structures, and show the bond formation to the carbon from the second resonance structure:
Or, we can show all electron motion in a single step:
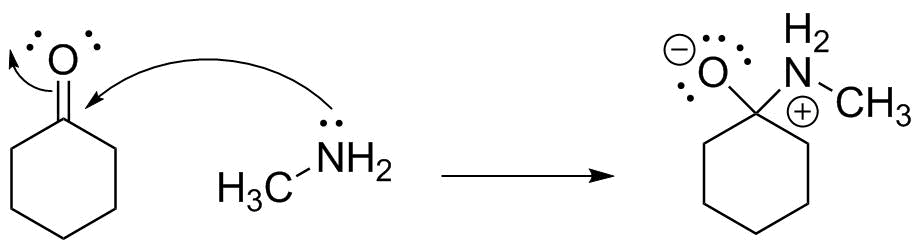
The final important aspect is to make certain the product is shown with the correct formal charges on the atoms. We are removing electron "ownership" from nitrogen, so it becomes positive, and adding electron "ownership" to oxygen, which becomes negative. Carbon is swapping one electron pair for another and so stays neutral. All other atoms are unchanged--meaning that you should be able to do this with ANY set of compounds having a nitrogen lone pair and a C=O bond.