Alkane nomenclature requires vocabulary that you will use throughout Organic Chemistry. This includes formal systems ("IUPAC" nomenclature) and names that have a historical origin without necessarily a systematic structural meaning ("common" names). Often, common names have been adopted by IUPAC as sytematic root names due to ease of describing a structurally complex system.
All organic compounds, alkanes especially, are named using a root name that reflects the number of carbons in the longest carbon chain:
| Carbons |
Root name |
Alkane name |
| 1 |
meth- |
Methane |
| 2 |
eth- |
Ethane |
| 3 |
prop- |
Propane |
| 4 |
but- |
Butane |
| 5 |
pent- |
Pentane |
| 6 |
hex- |
Hexane |
| 7 |
hept- |
Heptane |
| 8 |
oct- |
Octane |
| 9 |
non- |
Nonane |
| 10 |
dec- |
Decane |
More complex structures have branched carbon chains. Naming these is more complex, but straightforward.
- Locate the longest contiguous carbon chain and name it--this is the base name of the compound. When there is a choice of two equivalent-length chains to provide the base name, choose the one that has the least number of substituents.
- Number the chain starting with the end nearest a substituent.
- Name each alkyl substituent based on the number of carbons in it.
- List multiple groups in alphabetic order.
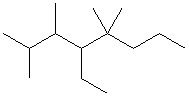
The first step is to find the longest carbon chain and count the number of carbons:
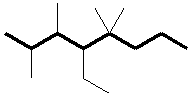
This is an octane (8 carbons). Number the chain so that the substituents have the lowest aggregate numbered positions:
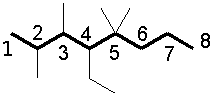
Designate the substituents, ordering them alphabetically in front of the base name:
4-ethyl-2,3,5,5-tetramethyloctane
The official IUPAC rules are found at https://www.acdlabs.com/iupac/nomenclature/; the direct link for branched hydrocarbons is https://www.acdlabs.com/iupac/nomenclature/79/r79_36.htm.