Core concept #1: the bare proton (H+) is good for bookkeeping but never exists in reality in chemical systems.
Protons can exist by themselves in interstellar space, and in the Sun, but when we we refer to "H+" we are using a formalism. The positive charge on the proton is attracted to anything with electrons and will bind to whatever it can find. In the gas phase, generation of bare protons (by electric discharge) in methane results in the CH5+ cation! However, we can (and do, often) illustrate proton transfer by showing "H+"--recognizing that it is in reality coming from some strongly acidic proton carrier.Core concept #2: the Brønsted acid concept revolves around the relative abilities of species to give up or accept protons.
We can define a proton transfer equilibrium reaction as follows (recognize that this is a formalism--since we are using "H+")
The equilibrium constant will reflect how easy it is for H-A to give up a proton:
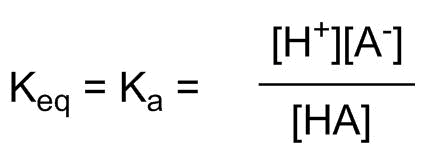
We are using the construct of a bare proton as the basis for a "ruler" to compare acidity of any H-A, and conversely, the basicity of any A-. Since many of these values are very large or very small, it is more convenient to use a logarithmic scale called pKa, defined as:
pKa = -log Ka
Core concept #3: the location of equilibrium for any proton transfer is determined by the Ka values
Let's take an example: dissolve HCl in water and describe the equilibrium that occurs. We'll analyze this in several steps:- Identify every acid and its conjugate base.
- Choose (arbitrarily) which acid will be the proton donor. (If the equilibrium is not favored, we can turn everything around.)
- Write the equation for Ka for the proton donor. Look up and list Ka.
- For the proton acceptor, write the reverse reaction for Ka for its conjugate acid. Look up Ka for the conjugate acid; Keq for the reaction as you wrote it is 1/Ka.
- Add the two reactions. You will see that the H+ term cancels out--if it does not, you made a mistake.
- The equilibrium constant is the product of the two Keq values, or more simply Ka(donor)/Ka(acceptor-conjugate acid).
- H-Cl is the proton donor, and H2O is the proton acceptor. The conjugate base of HCl is Cl-, and the conjugate acid of water is H3O+.
- H-Cl ⇋ H+ + Cl- Ka = 108 (pKa = -8)
- H+ + H2O ⇋ H3O+ Keq = 1/50 (pKa = -1.7)
- H-Cl + H2O ⇋ H3O+ + Cl- Keq = 108/50 = 2 x 106
If we know Ka (or pKa) values--or can estimate them--we can quickly predict proton transfer equilibria. You should look at Table 2.2 carefully; I strongly recommend memorizing the following 6 pKa values. In any proton transfer analysis you then have merely to estimate which of your memorized compounds any of the proton donors or conjugate acids most closely resembles.
Compound
pKa
Comments
Hydronium
Acetic acid
Ammonium ion
Water
Acetylene
Ammonia
Ethylene
Methane
Core concept #4: Stablilizing charge and/or electrons on either side of the equation will shift the equilibrium toward that side.
We will be looking at a variety of ways that a structure will stabilize charge and electrons. In general you should look at the following:- Electronegative atoms or groups will stabilize electrons and negative charge (in the conjugate base), and destabilize positive charge (in the conjugate acid). Makes a stronger acid. This applies to both the atom losing a proton, and when that atom is close to other electronegative atoms.
- Increasing atom size will stabilize electrons and negative charge (in the conjugate base). Makes a stronger acid.
- Electron delocalization (illustrated by multiple resonance forms) stabilizes electrons and negative charge (in the conjugate base). Makes a stronger acid.
- Resonance stabilization of a positive charge is also possible and will make for a weaker conjugate acid. Electron-donating groups will also weaken any conjugate acid.