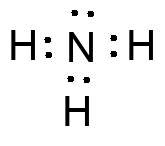
Look at the ESP map to see that the nitrogen has a negative potential (red) and will be attracted to areas of positive charge and electron deficiency.
Another common example, this time of a Lewis base. The lone pair leads to a pyramidal molecular shape, and th
e ability of N to support a formal positive charge allow ammonia (and amines) to share the lone pair readily.<
br>
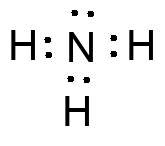
Look at the ESP map to see that the nitrogen has a negative potential
(red) and will be attracted to areas of positive charge and electron deficiency.