Resonance structures are a critical component of how we communicate ideas about structure and stability. It is very important that you:
- Understand what resonance structures mean about bonding and structure;
- Know how to correctly draw resonance structures;
- Know when you need to consider and use an alternative resonance structure.
Correctly drawing resonance structures requires several steps:
- Identify the complete π system that you consider. This means looking for the longest run of double bonds on alternating (not adjacent) bonds, and then looking to see whether there are other adjacent features that (given the right hybridization) might participate in resonance: lone pairs, unpaired electrons (radicals), or electron deficient atoms.
- Draw a valid Lewis structure (you may have already started with one).
- Move a π electron pair in a double bond on the end of
the system to either: (a) the terminal atom of the π system or
(b) between that terminal atom and an adjacent, electron-deficient atom
to form a new π bond.
- Adjust the formal charges on atoms where you change electron count. If a new π bond forms to an electron deficient atom, the formal charge decreases by one. If the atom gives away its participation in a π bond, the charge increases by one. If a lone pair turns into a π bond, the charge also increases by one.
- Repeat for every additional double bond in the system.
- You can couple multiple electon-pair shifts if you
wish.
- Radicals are a little different since we rearrange them one electron at a time (see Chapter 3).
Examples
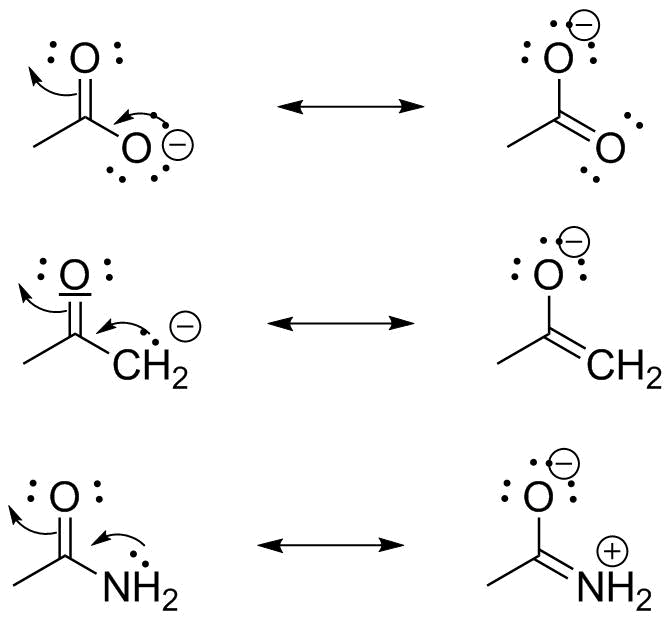 |
These are all effectively the same system: a lone pair delocalized into an adjacent C=O π bond. In all of these, I have avoided writing a "missing" resonance structure with a positive charge on the central carbon. Note that these 3 are all isoelectronic. |
|
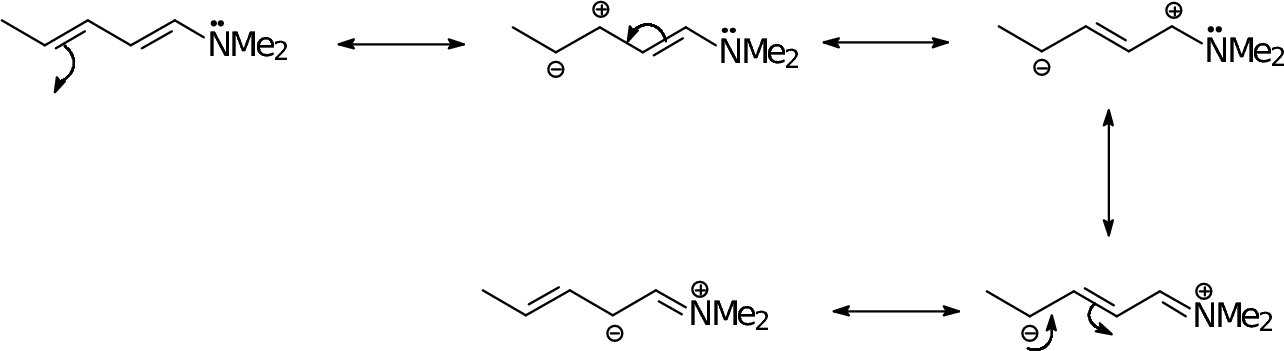 |
In an extended pi system, we can start the process from either end. |
|
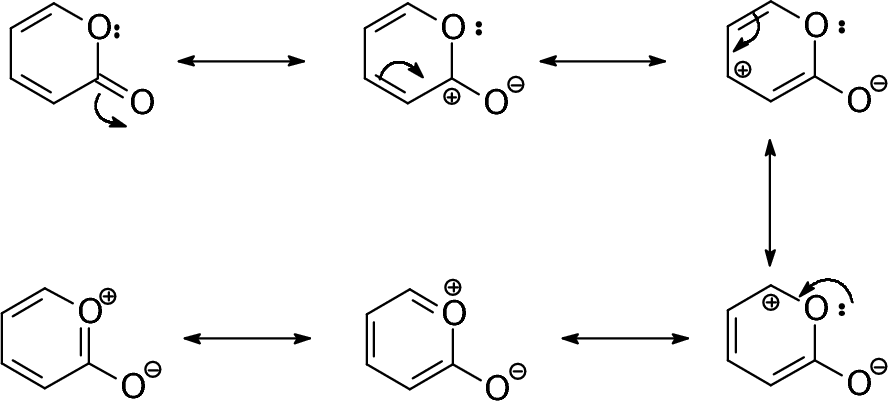 |
A cyclic example. |
|
Here are some common mistakes: AVOID THEM!
Adding electrons to an atom with a full octet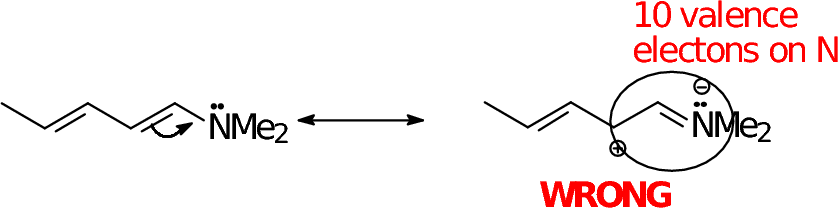 |
Adding electrons to an atom with a full octet when another electron pair can be "bumped" somewhere else. |
Ignoring saturated centers
 |
Not drawing all possible resonance forms; often a judgment call about relative importance.
|
Forgetting charges |
Omitting new charges |
You can probably tell by now that the most common mistakes to avoid all deal with either not drawing, or not understanding, proper bond-line and/or Lewis structures.