First, the rules for resonance structures:
- All resonance forms must be (or correspond
to, as in the bond-line formalism) valid Lewis structures.
- Different resonance forms represent the
same molecule: all nuclear positions are identical in the two
forms. What changes is the "formal" location of electrons.
- The number of unpaired electrons must remain the same.
- We look for "stability" in the resonance forms, as judged by:
- Greatest number of formal bonds
- Greatest number of atoms with complete octets
- Least amount of formal charge, and the most negative formal charge on the most electronegative atoms (O, N, S)
- Delocalization of charge over two or more atoms, and delocalization of bonding, is an important stabilizing factor in judging a set of resonance forms.
Molecular orbitals provide what initially looks like a very different picture. They are intrinsically delocalized descriptions, and much of the qualitative picture we get from resonance forms is tied up in the numerical results: MO energies, atomic charges. However, the shapes of the HOMO (Highest Occupied Molecular Orbital) and the LUMO (Lowest Unoccupied Molecular Orbital) along with the ESP map provide a qualitiative presentation of molecular properties and reactivity. It helps to make some comparisons of the descriptions from both directions.
We can now compare some examples of increasing complexity.
Page controls:
Methyleneiminium Cation
| Resonance structures |
Comments |
|
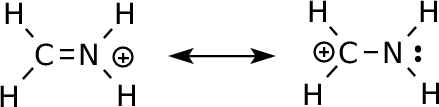 |
In one of the
structures, C is electron deficient (6 valence electrons) but in the
other, the more electronegative atom (nitrogen) has the positive
charge. We expect that the charge is shared between the atoms,
but have no way of knowing how much. |
|
|
Show the HOMO: occupied; E=-0.656 Show the LUMO: empty, E=-0.326 Show the electrostatic potential map(red/orange = negative, green/blue = positive) Download methyleneiminium.pqr(no MO data) |
From the MO
computation, the results tell us the charges on the atoms are: carbon: +0.22 nitrogen: -0.35 C-H: +0.18 N-H: +0.38 Note a couple things in the MO shapes: the HOMO is largest on the nitrogen--the most reactive electrons are there (or, the pi bond is polarized toward nitrogen). This corresponds to what the right-hand resonance form describes. The LUMO is oriented toward the carbon. Anything with electrons will interact at this end of the pi bond. The delocalization of the pi bond is consistent with the left-hand resonance form: this molecule will have a barrier to rotation about C-N (and this should be about 80 kcal/mol). Because this molecule has a positive charge, the LUMO offers the more relevant picture of reactivity: it will react with things that have (at least partial) negative charge--and therefore electrons--which will interact with the LUMO to form a new set of MOs. |
Acetate Anion
| Resonance: |
Comments |
|
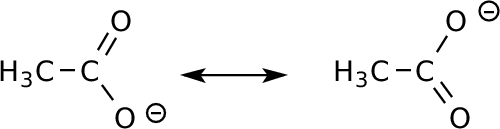 |
Charge shared between
two oxygens. Because of symmetry, expect the charge to be equally
distributed between the two. |
|
| LUMO: E = 0.253 Show the LUMO (empty), E=+0.253 Show the π HOMO (occupied), E=-0.012 Show a lone pair MO Show the π bonding MO, E=-0.139 Show the electrostatic potential map Download acetate.pqr(no MO data) |
Atomic charges from the MO calculation: Carboxyl C: +0.80 O: -0.79 Methyl C: -0.22 H: 0 The LUMO is not very relevant, but you can see the antibonding interaction between C and the two Os. (Other contributions from the methyl mix in.) Here, the most relevant MO is the HOMO, because the molecule is negatively charged, and will react with positively-charged things seeking the most reactive electrons. We do need to worry about the distinction between the pi electrons and (nonbonding) lone pairs: the latter are higher in energy, but all of the pictures give the same idea: reactive electrons are on the oxygen. A low-lying MO gives us the idea of delocalization across the carboxylate. |
Methyl vinyl ketone (but-3-en-2-one)
| Resonance |
Comments |
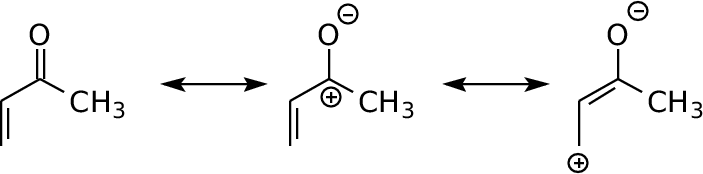 Show the LUMO (empty), E=+0.047 Show the highest π MO (occupied), E=-0.072 Show the lower π MO (occupied), E=-0.365 Show the electrostatic potential map Download MVK.pqr(no MO data) |
Resonance can
place a
negative charge on the most electronegative atom (O). The
multiple bonding allows the corresponding positive charge to be
delocalized between Carbon-2 and Carbon-4. These properties are
imposed at the cost of creating partial electron deficiency at these
carbons. There is partial double bond character between Carbon-2
and Carbon-3. The ESP map (click on link) shows the polarization of the molecule and the increased positive electrostatic potential at carbons 2 and 4 (blue/green). Note the positive potential at the methyl protons as well. The LUMO illustrates the electrophilic properties of Carbons 2 and 4. The HOMO and HOMO-3 show the delocalization of pi bonding, particularly between Carbon-2 and Carbon-3 The lone pairs on oxygen are comparable in energy to the HOMO, accentuating the electron-rich nature of the oxygen. |