The molecular orbital description of bonding in methane does several things for us.
- It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach.
- It should tell us (quantitatively) about the energies of different electrons
- This energy description should reproduce experimental findings that two of the valence electrons have a lower energy than the other 6.
The bottom 4 are all occupied MOs, and lower in energy than the non-bonding energy level (0 eV). Note that the lowest energy MO is evenly distributed around the molecule: these two electrons are equally shared between carbon and all 4 hydrogens. The next three MOs (2,3,4) are all of the same energy, and differ only in their orientations. There is a node (a phase change: blue to red) that decreases the net bonding, but note that for each MO, there is an area of electron density between carbon and each of two hydrogens. All of the MOs are delocalized, but all of them show high electron densities between carbon and hydrogen. If we mathematically added out sp3 C to hydrogen 1s valence bonds, we'd get the same thing: MO (1) = bond 1 + bond 2 + bond 3 + bond 4 MO (2) = bond 1 - bond 2 MO (3) = bond 1 + bond 2 - bond 3 - bond 4 MO (4) = bond 3 - bond 4 |
The highest four MOs are empty (so they don't affect energy) and have a
phase change between C and H. If we were to put an electron in
one of these MOs, that would decrease the bonding between carbon and
the hydrogens. Click on a link to visualize each molecular orbital. Show the 8th MO: E=+3.90 eV Show the 7th MO: E=+3.90 eV Show the 6th MO: E=+3.90 eV Show the 5th MO: E=+1.99 eV Show the 4th MO: E=-10.58 eV Show the 3rd MO: E=-10.58 eV Show the 2nd MO: E=-10.58 eV Show the lowest energy MO: E=-18.79 eV Here is an energy level diagram showing how the 4 hydrogen 1s orbitals and the 4 carbon atomic orbitals (2s and three 2p) mix to make 4 bonding and 4 antibonding molecular orbitals: 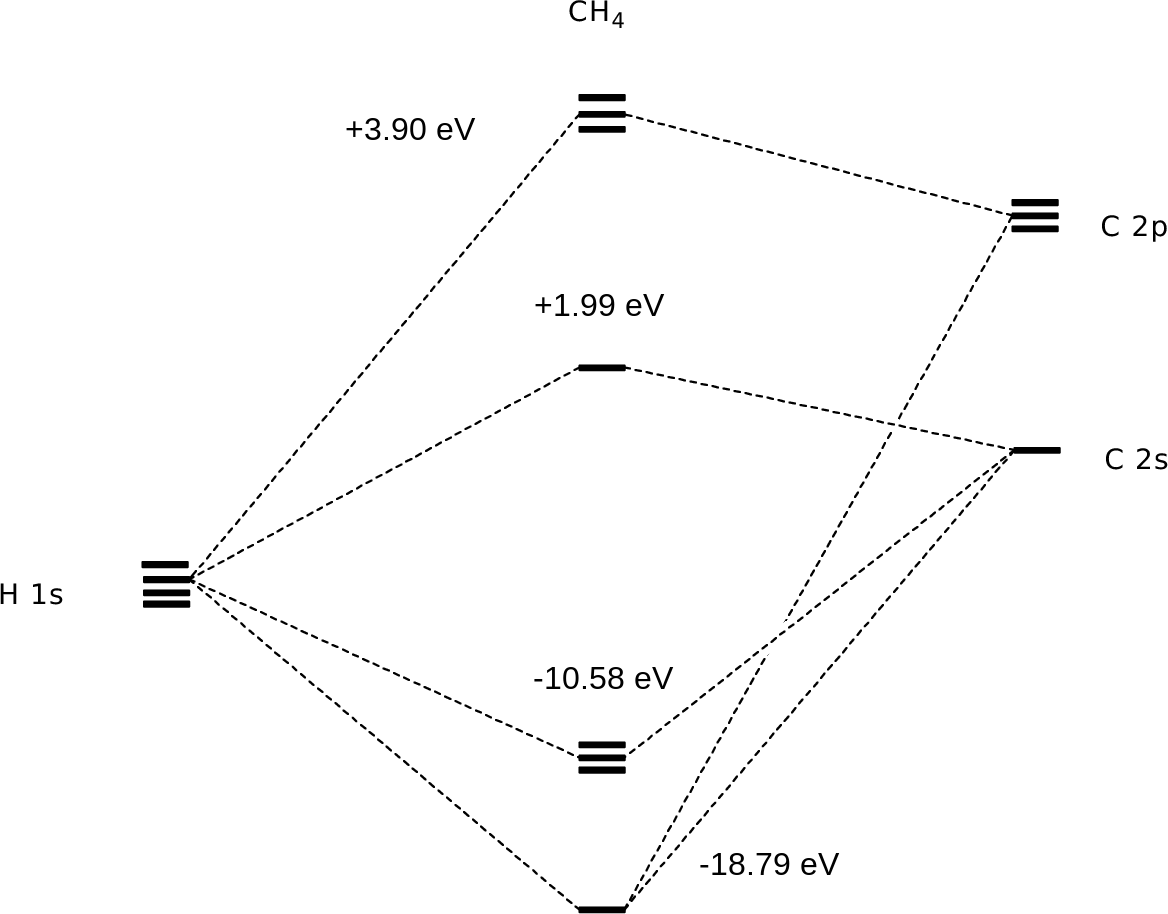 (calculated at the B3LYP/cc-pvdz level using Jaguar, version 7.8, Schrödinger, LLC, New York, NY, 2011. See also Gelius, U., in Electron Spectroscopy, pp. 311-314. D. A. Shirley, ed., American Elsevier, New York, 1972.) |