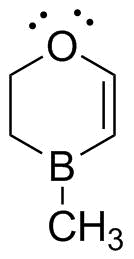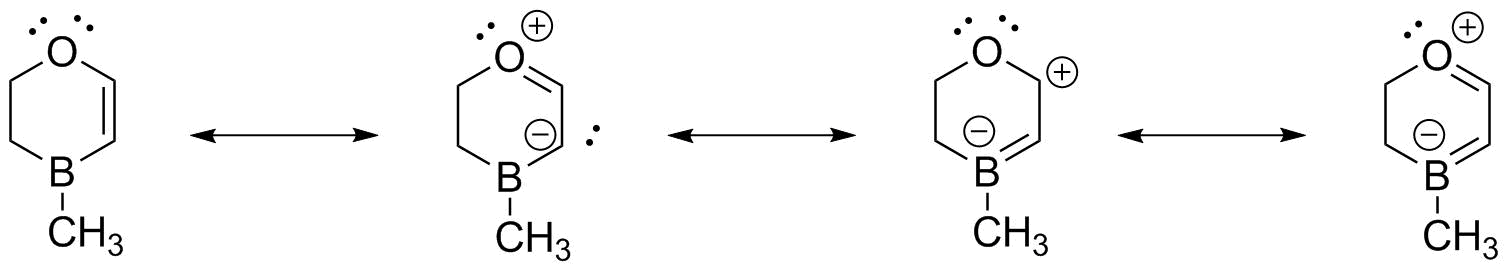
The MO description requires that we recognize that all 4 contiguous atoms can be sp2 hybridized. These can then overlap and interact to form a set of 4 MOs--two bonding and two antibonding.
We can illustrate these in the 3-D model (Boron is the pink atom); the HOMO and LUMO illustrate the point:
|
info
clear
no info
|
Show the constituent pz AOs. Show the HOMO Show the LUMO |