Problem: Geometry & Hybridization
Determine the geometry of the atom indicated with the arrow and state its atomic orbital hybridization.
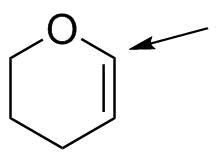
Analysis
We must first translate this picture into a complete structure, taking into account the "shortcuts" involved in the bond-line structure shown. This involves several steps to remember:
- The vertices at the end of all bond lines represent carbon unless the atomic symbol is explicitly shown.
- All carbons will have a total of four bonds; therefore, any bonds not explicitly shown must be to hydrogens not shown.
Given this, the carbon atom to which the arrow points is shown having three bonds: a single bond to the oxygen, and a double bond to the carbon on the other side. The missing 4th bond must be to hydrogen.
A full representation is shown below.
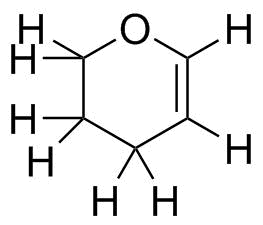
Now, we have to predict the geometry of that carbon. Carbon in most compounds will have only three possibilities: tetrahedral, trigonal planar and linear. Since it is connected to 3 other atoms (one O, one C and the H--disregard multiple bonds), it
must be trigonal planar. This also provides us the atomic orbital hybridization: trigonal planar carbon is sp2 hybridized; the unhybridized p orbital is used to make the multiple (π) bond.
Draw the molecule below and look at the 3-D structure.

