 |
PHILIP R. WATSON, PROFESSOR
B.A. Oxford University, 1974
Ph.D. University of British Columbia, 1979
Department of Chemistry, Oregon State University,
Corvallis, Oregon 97330-4003, USA
Phone : (541)-737-6740 Fax : (541)-737-2062
E-mail : watsonp@chem.orst.edu |
|
P-Chem CD
About P-Chem
System requirements
Structure of P-Chem
modules
Table of contents
of P-Chem
How to get P-Chem
Download a
demo version of P-Chem
About P-Chem
This highly visual, interactive program is designed to help students better understand
the role of molecular characteristics and environmental variables on physical properties.
All of the major topic areas of physical chemistry - thermodynamics, quantum chemistry,
statistical thermodynamics, kinetics and reaction dynamics, polymers, solid state and
surfaces - are covered in 40 individual modules.
Students can interactively investigate the effects of varying system parameters, such
as temperature and pressure, over a wide range. Generally, they can select from a range of
predefined molecules of various types, or enter the data for a molecule of your choice.
The results of changes in molecular properties or system parameters are immediately
reflected in colorful plots, animated simulations and movies. In this way they can
formulate trends and make predictions (useful for home work assignments). Accompanying
each module is explanatory material that briefly reviews the underlying theory, using
hyperlinked definitions and biographies.
Included in this text is a question and answer session so students can test their
understanding of the material.
System requirements
- PC running Windows 3.1 or higher
- 6 MB disk space
- 256 color video card
- mouse
Table of
contents of P-Chem
Thermodynamics
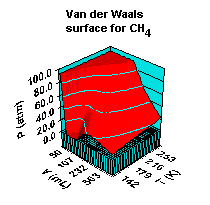 Real gases - Van der Waals equation of state
Real gases - Van der Waals equation of state
Thermochemical enthalpy cycles
3rd Law entropies
Equilibrium
- Van’t Hoff equation
- Clausius-Clapeyron equation
- ideal solutions
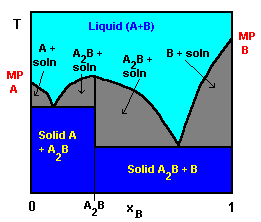 - phase diagrams
- phase diagrams
Debye-Huckel law
Quantum chemistry
Simple systems
- particle in a box (1-D, 2-D)
- harmonic oscillator
- rigid rotor
- tunneling through a barrier
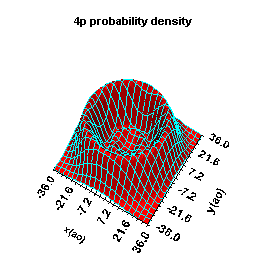
Hydrogen atom
- radial functions/energy levels
- wavefunctions
Polyatomic molecules -Huckel theory
Spectroscopy
- rotational spectra of diatomics
- vibrational spectra of diatomics
- IR/Raman spectra of polyatomics
- NMR - spin-spin coupling
Intermolecular forces
Statistical thermodynamics
Boltzmann distributions
- rotational levels
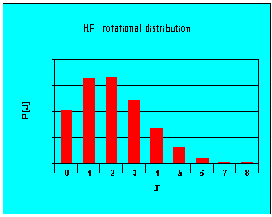
- vibrational levels
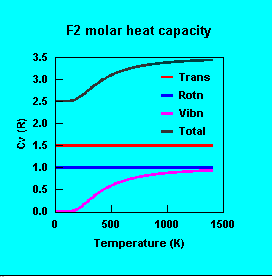
Heat capacities of small molecules
Kinetic theory of gases
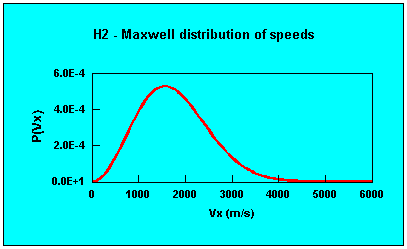
Distribution of velocities (1-D)
Distribution of speeds
Molecular collision frequency
Flux on surface
Chemical Kinetics
Rate laws
- elementary reactions & order plots
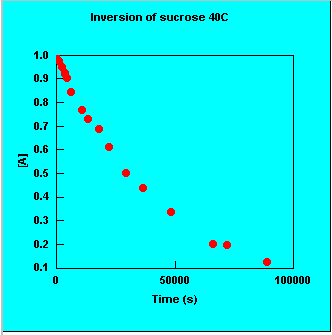
- multistep first-order reactions
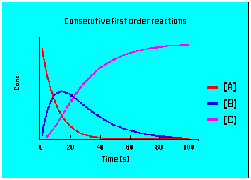
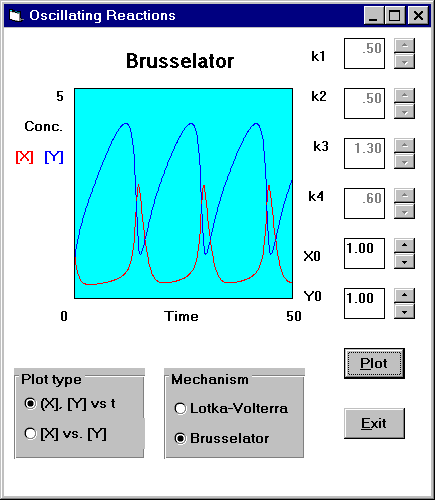
Diffusion
Oscillating reactions
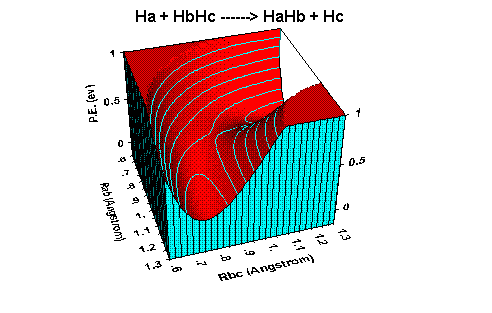
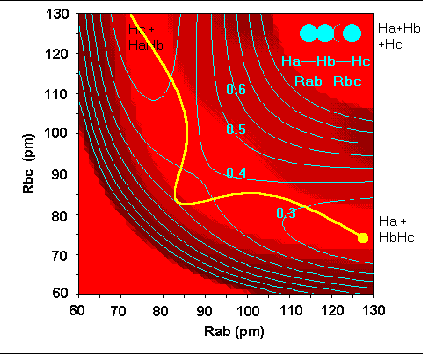
Reaction dynamics - PE surface for H + H2 reaction
Macromolecules
Chain configuration
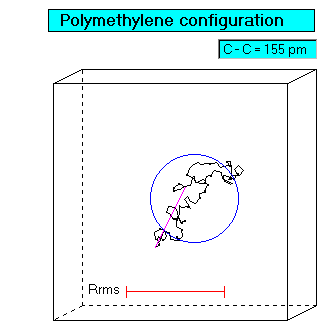
Molar mass by viscosity/osmometry
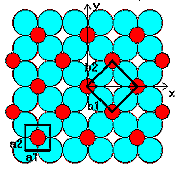
Solid state
Lattice types
Miller indices
X-ray diffraction
- Bragg equation
- Fourier analysis
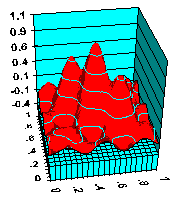
Surfaces
Surface structures
Adsorption - Langmuir/BET isotherms
Structure of P-Chem
modules
Each module in P-Chem has a standard structure in which the screen is split into two
areas called the Text and Work windows.
The Text window provides :

Background - a a quick-reference summary of the underlying
theory with hypertext links to definitions, to explanations of related concepts, and to
biographical/historical information

Explorations - suggestions of useful parameter variations to
try in the Work Window

Questions and Answers - mainly conceptual tests of student
understanding of basic principles
In the Work window students can :
- choose molecules/systems of interest from lists (or enter their own data)
- set the values of environmental variables like temperature and pressure
- use the mouse to indicate important locations in a graphic - for
instance, a spectrum or phase diagram
- choose the type of plot (e.g. 2D vs. 3D) or the method of data treatment
(e.g. the reaction order to fit to rate data)
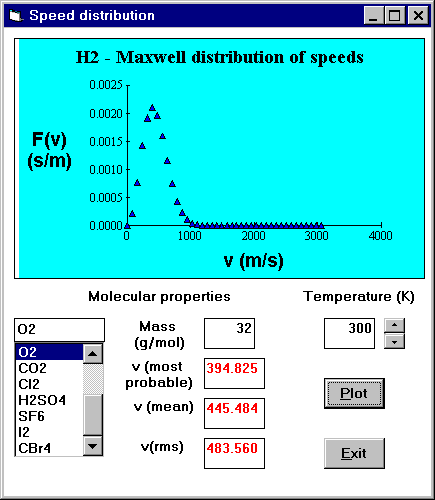
In most modules students can choose from list of predefined molecules, solids etc. If
they want to investigate a different system, in general they can enter the relevant data
directly into the appropriate boxes. In some cases, such as the modules dealing with
adsorption isotherms or rate equations, they
choose,
or provide, a data file of real experimental data. The values of environmental and
molecular variables are displayed in boxes. When a student changes an environmental
parameter, the molecular parameter is updated e.g. altering the temperature will cause the
mean speed of a molecule to update.
How to get P-Chem
P-Chem is sold by John Wiley and Sons, College Division.
Download a
demo version of P-Chem
If you would like to download a demo version of P-Chem
- Click here
http://www.chem.orst.edu/PersonalHomePages/watson/pchem/pchem.htm
Last Updated by Philip R. Watson on Monday, September 09, 2002


 Real gases - Van der Waals equation of state
Real gases - Van der Waals equation of state - phase diagrams
- phase diagrams















