Red is 0.4 positive and Green is 0.4 negative.

| Friedel-Crafts Reaction
of 4-Bromobenzoylchloride and Benzene using aluminum chloride catalyst (not shown). Red is 0.4 positive and Green is 0.4 negative. |
||
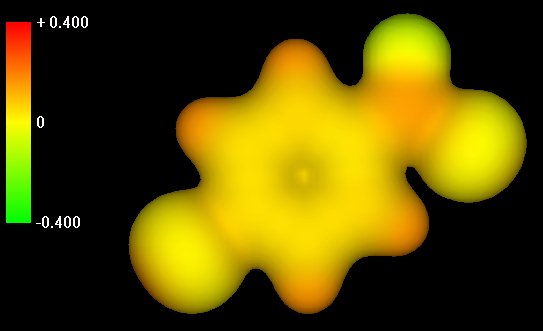
| SlSlide 1: Charge distribution in
acid chloride reagent.
|
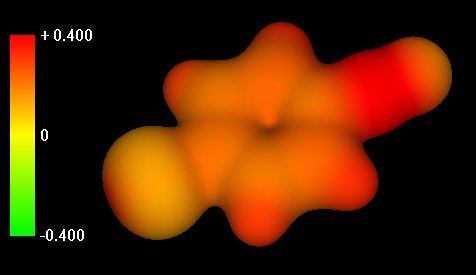
Slide 2: Acylium ion. Charge
distribution in the acylium ion that has formed from the action of the catalyst on the
acid chloride. Notice increase in positive charge throughout structure, especially
concentrated on the previously carbonyl carbon.
|
|
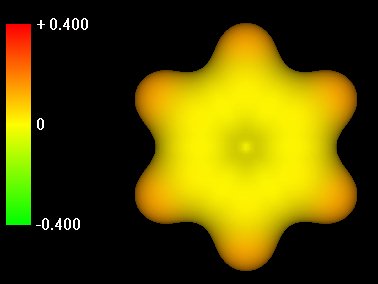
| Slide 3: Benzene reagent.
|
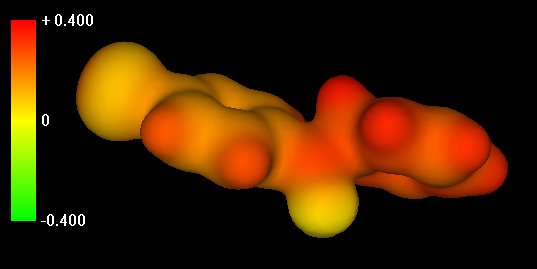
Slide 4: Sigma Complex
intermediate formed by attack of the electrophilic acylium ion on benzene. Note the
hydrogen at the tetrahedral center accessible for removal by AlCl4-.
|
|
| Slide 5. A
substituted benzophenone is the final product with the production of HCl and the
regeneration of the catalyst, AlCl3.
|
||