Preparation of Methyl Cyclohexanecarboxylate
The chemistry you will use is outlined below:
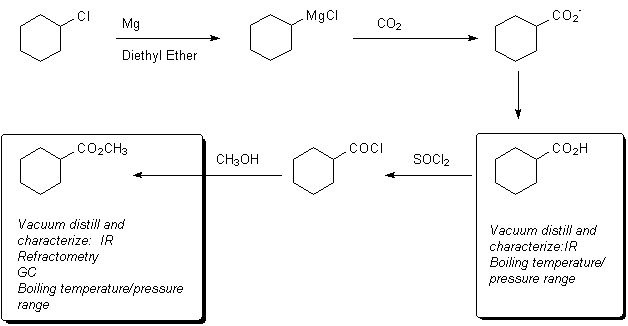
A useful background paper on the mechanism of Grignard reagent formation--particularly the origin of radical coupling products--is here..
You should be aware of several safety issues.
- The chlorocyclohexane is of the same chemical class as iodomethane, so keep it in the
fume hood, try not to spill it, and use gloves throughout the experiment.
- The Grignard reagent is a strong base and very caustic; your gloves again serve as
protection for your hands. The reagent is also quite air-sensitive, and ether is
quite flammable. Be sure to get help from a TA when doing the transfer from the
reaction flask back into the addition funnel!
- The organic compounds used in this experiment are volatile and have disagreeable odors,
so avoid keeping open containers of any of these outside the fume hood.
Grading:
100 points (out of 200 total for the report) are possible for "Results." You will be graded on yield, purity
and characterization you relate in Report I.
- You should calculate the yield of pure carboxylic acid from chlorocyclohexane.
- You should calculate the yield of methylcyclohexanecarboxylate from
cyclohexanecarboxylic acid.
- You should then calculate the overall yield, which is the product of the two
individual yields.
- Your refractive index for the ester should match the literature value (1.4408 ± 0.0002
at 25°C); an acceptable temperature-corrected value will be within ±.0005 of this.
- You should take IR spectra of both the carboxylic acid and the ester; you need to
identify any peaks in the functional group region, and establish that the fingerprint
region matches that of a reference spectrum.
- You should demonstrate with gas chromatography that the purity of the liquid ester is
>99%.
- You will need to report the boiling range and pressure for each compound; this, like
melting points of solids, is a characteristic physical property.

