
CH 334 Practice Final
1. Naming (12 pts)

Ethyl propyl ether
E-6-methyl-2-heptene
meso-3,4-dihydroxyhexane a tertiary alcohol with 5 carbons

2. Rank the following structures according to increasing pKa: (5 pts)

lowest pKa I<III<II <V<IV highest pKa
3. Draw the three staggered Newman projections of the C2-C3 bond of (2S,3S)-2,3-difluoropentane. Indicate which is the lowest energy conformation, and which is the highest energy conformation. (hint: The methyl group is larger than a fluorine atom) 15 pts
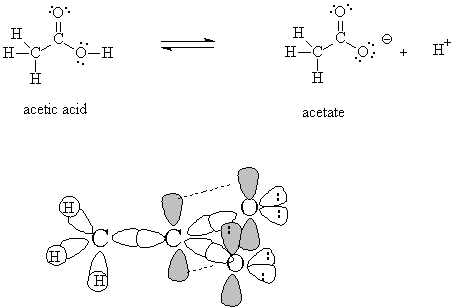
4. Acetic acid is a carboxylic acid with the condensed formula CH3CO2H. The conjugate base of acetic acid is called acetate. (20 pts)
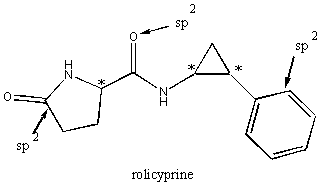
5. Rolicyprine is a synthetic antidepressant sold under the trade name Cypromin, and has the structure shown below. (12 pts)
6. State the stereochemical relationship between each of the following structures: (12 pts)
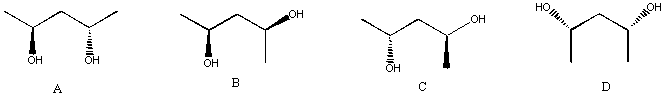
A and B same B and C diastereomers
A and C diastereomers B and D diastereomers
A and D diastereomers C and D same
7. Give the major product you expect to form from each of the following reactions. Indicate all relevant stereochemistry: ( 3 pts each)
** Please donít do question C, this reaction ( the anti- Markovnikov addition of HBr) has not been covered this term.
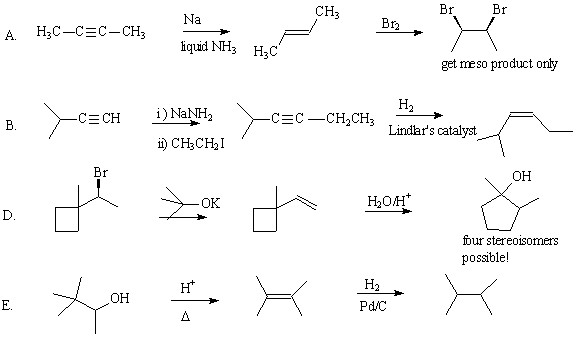
8. When the alkyl halide A is treated with potassium t-butoxide, an E2 reaction occurs to form one product. When B is treated with the same reagent, the E2 reaction gives two products. (12 pts)
OOPS! There is a mistake on this question. It is compound A that gives two products, and compound B that gives only one product. For part II, please show the E2 mechanism on structure B.

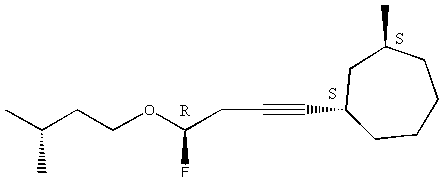
9. Assign absolute configuration to each stereocenter in the following molecule.
10.Rank the following carbocations in order of
increasing stability. ( 5 )
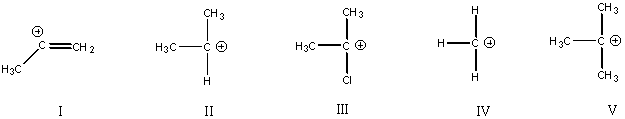
least stable IV< I<II<III<V most stable
11. When the alkene below is treated with HCl, methylchlorocyclohexane is one of the products formed. Give the complete arrow pushing mechanism for this reaction. (10)
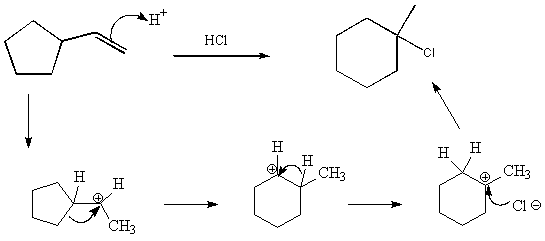
12. Give the complete arrow pushing mechanism for the following reaction: ( 8 pts)
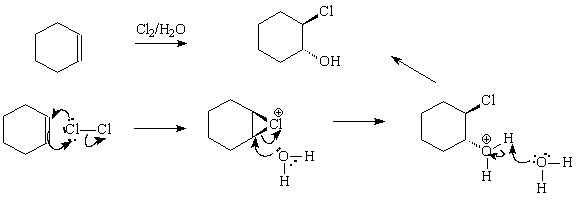
13. Mystery molecule. From the clues given below, deduce the structure of mystery molecule, draw it and name it. (8)
a) It has the formula C6H10
b) It has two methyl groups
c) When treated with H2 in the presence of the Lindlarís catalyst, no reaction occurs.
d) When treated with H2 in the presence of a Pt catalyst, it forms a single meso compound with two stereocenters.
e) When treated with Br2/CH2Cl2, it forms a racemic mixture of enantiomers

1,2-dimethylcyclobutene