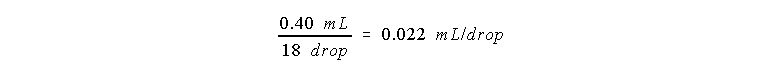
Examples of Vitamin C Experiment
Calculations
The data used in these example calculations are imaginary. Do
not assume they are representative of the results you obtained in
lab.
Example 1.
Suppose your micro buret requires 18 drops to fill the 0.40 mL
well. Then the volume of one drop is
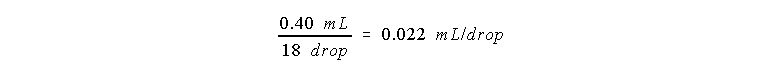
Example 2.
Suppose 2 drops (from your calibrated micro buret) of a
standard 0.50 mg Vit-C per mL solution requires 7 drops of DCP to
reach the end point. Then 2 drops of an unknown Vit-C solution
are titrated and suppose 8 drops of DCP are needed.
The concentration of Vit-C, [Vit-C], in the unknown solution
will be in proportion to how much DCP is needed:
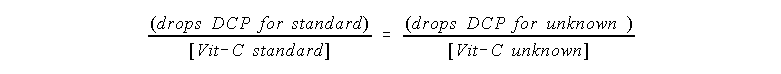
Plugging in the values:
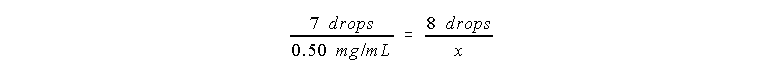
So x = 0.57 mg/mL in the unknown.
Example 3.
Now suppose that a whole fruit is to be analyzed. If the whole
fruit contains 12 sections, and one section is squeezed until it
yields 6.5 mL of juice, and 2 drops of that juice is titrated
with 9 drops of DCP, how many mg of Vit-C are in the whole fruit?
First figure out the concentration of Vit-C in the juice the
same way as in example 2:
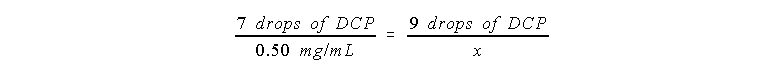
Then the concentration of Vit-C in the juice is x = 0.64 mg/mL
Now this is the concentration of Vit-C in the 2 drops of
juice. We know how many mL come from each segment, and we know
how many segments are in the whole fruit. So we can calculate the
mg of Vit-C in the whole fruit:

= 50 mg Vit-C per whole fruit
This is how much Vit-C was in the whole (imaginary) fruit.
Your results may differ.