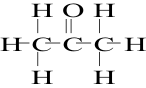
Exam 2 CH 130 Name ________________________
May, 2001 Circle your name if you want to pick up graded exam personally
There are 5 pages and 25 questions on this exam. Be sure you have them all.
Write your answers to the multiple choice questions in the numbered boxes on the last page of this exam.
1. Amines can be considered organic derivatives of the inorganic compound
A. ammonia
B. carbon dioxide
C. sodium hydroxide
D. water
E. None of these
2. Which formula correctly illustrates the form which acetic acid would take in basic solution?
3. The carbonyl group is
A. a general term for any functional group involving a carbon-oxygen bond
B. found only in aldehydes and ketones
C. a functional group in which carbon and oxygen are joined by a double bond
D. a functional group with a 6-membered ring where at least one atom is oxygen
E. produced by reduction reactions of primary or secondary alcohols
4. Members of which class of biomolecules are the building blocks of proteins?
A. glycerols
B. monosaccharides
C. fatty acids
D. amino acids
E. nucleic acids
5. When a nitrogen atom in an organic compound has four covalent bonds, the compound is called a
A. primary amineB. secondary amineC. tertiary amineD. ammonium ionE. tetraamine
6. When an alcohol reacts with phosphoric acid, the product is referred to as a
A. phosphate salt
B. phosphate ester
C. phosphate ion
D. pyrophosphate
E. phosphol
7. Which of the following is a use of formaldehyde?
A. flavoring
B. hormone
C. preservative
D. sweetener
E. solvent
8. Polar side groups of amino acids, along with acidic and basic side groups, are said to be _________ because they are attracted to water molecules.
A. hydrophilic
B. hydrophobic
C. ionized
D. unreactive
E. None of these
9. All of the following concerning a three-carbon ketone are true EXCEPT
A. Its condensed formula is CH3-CO-CH3
B. Its systematic name is propanone
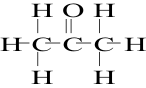
C. Its common name is acetone
D. Another acceptable name is methyl ethyl ketone
E. Its structural formula is ......................................................>
10. When an alcohol reacts with a carboxylic acid, the major product is
A. an amide
B. an amine
C. an ester
D. a salt
E. a soap
11. When an amine behaves as a base it ______ a hydrogen ion to form a(an) _______ ion.
A. gains ; hydronium
B. gains ; ammonium
C. loses ; hydroxide
D. loses ; hydronium
E. loses ; ammonium
12. Which structure represents a zwitterion?
A.
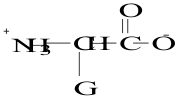 B.
B.
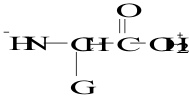 C.
C.
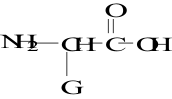
D.
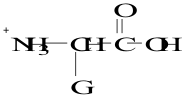 E.
E.
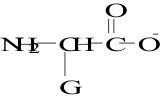
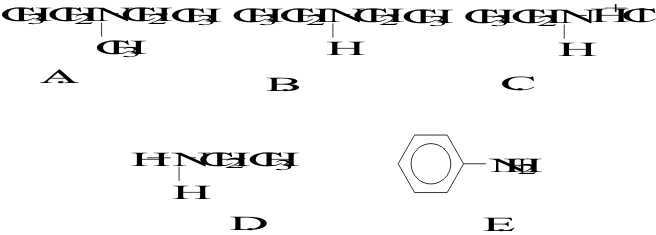
13. Which of the following molecules is a tertiary amine?
14. Which carboxylic acid is used to prepare the compound:
A. CH3-CH2-COOH B. CH3-COOH
C. CH3-CH2-CH2-COOH D. (CH3)2-CH-CH2-COOH E. H-COOH
15. Which of the following statements about Nylon is TRUE?
A. Nylon is a polyaldehyde
B. Nylon will not dissolve in strong acid or base
C. Nylon is the product of the reaction between a dicarboxylic acid and a dialcohol
D. Nylon is the product of the reaction between a dicarboxylic acid and a dialdehyde
E. The separate polymer strands in Nylon are held to each other by hydrogen bonds
16. What is the product of the reduction of butanal?
A. butane B. 2-butanol C. butanoic acid D. 1-butanol E. no reaction
17. Two functional groups that are present in all amino acids are the _____ group and the _____ group.
A. hydroxyl ; amide
B. carbonyl ; amide
C. carboxyl ; ester
D. acetal ; amino
E. carboxyl ; amino
18. What makes an amino acid “non-essential?”
A. Humans do not need it to live; only plants need it
B. The human body can make it as needed from other chemicals
C. It prevents the proper operation of a living system
D. The human body must acquire it through diet
E. It cannot bond to other amino acids
19. The bond between amino acid residues in proteins is called the
A. ester link
B. amide attachment
C. peptide bond
D. hydrogen bond
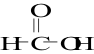
E. hemiacetal bond
20. What is the common name of the molecule shown here:
A. acetic acid
B. ascorbic acid
C. formic acid
D. lactic acid
E. oxalic acid
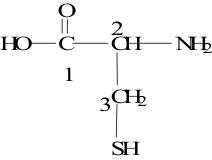
21. Consider the molecule shown to the right. Which carbon is chiral?
A. 1
B. 2
C. 3
D. 2 and 3
E. none of these choices
22. Which of the following would be the most toxic to humans?
A. acetic acid B. formaldehyde C. ethyl acetate D. acetone E. ethanol
23. What is the name of the major product of the reaction of ethanol and butanoic acid
24. Draw an amino acid that would be considered from the non-polar (or alkyl) group.
25. Acetaminophen and ibuprofen are shown below. Identify which is acetaminophen by its ability to form extended chains via hydrogen bonds which cause acetaminophen to have a relatively higher melting point than ibuprofen. Indicate clearly the groups involved in the hydrogen bonds.
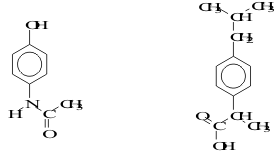
Multiple choice answers: put the correct letter in each box
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
# Multiple.Ch.______ + Points from 24+25+26 ______ = _______ ×4 = Total Exam Points ________