Final Exam
CH 130
June, 2000
There are 6 pages and 37 questions on this exam. Be sure you have them all.
Write your answers to the multiple choice questions in the numbered boxes on the last page of this exam. Each of the 36 multiple choice problems is worth 2.5 points. Problem 37 is worth 10 points.
1. Which type of dye would be expected to have the greatest color fastness through multiple washings?
A. Vat dyes (such as indigo)
B. Acid dyes
C. Basic dyes
D. Reactive dyes
E. All are equivalent
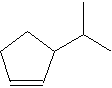
2. In the molecule shown to the right, the number of C
atoms is ______ 
and the number of H atoms is _______.
A. 6, 12
B. 6, 14
C. 8, 14
D. 8, 12
E. None of these
3. Which of the following molecules is chiral?
A. CH3OH B. CH3CH2OH C. NH2CH2COOH
D. CHCl2Br E. NH2CH(CH3)COOH
4. A mutation involves an error in
A. mRNA manufacture
B. translation
C. tRNA manufacture
D. DNA replication
E. transesterification
5. The functional group illustrated by the molecule shown
to the right is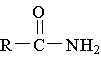
A. alcohol B. amide C. ether D. ester E. amine
6. What is the IUPAC name of the compound CH3-CH2-COOCH3 ?
A. 2-butanoic acid
B. 3-butanoic acid
C. methylethanoate
D. propylmethanoate
E. methylpropanoate
7. When an alkane reacts with an element from group 7A, the reaction is referred to as
A. combustion
B. decomposition
C. displacement
D. halogenation
E. oxidation
8. The name of the polymer formed from CH2=CH2 is
A. polyethylene B. polypropylene C. polystyrene D. polyvinyl chloride
E. None of these
9. Which of the following is the most soluble in water?
A. diethyl ether B. methanol C. 1-butanol D. 1-decanol E. n-decane
10. All the following are true concerning a two-carbon aldehyde EXCEPT
A. Its condensed formula is CH3-CHO
B. Its systematic name is ethanal
C. Its common name is acetaldehyde
D. Its structural formula is shown to the right: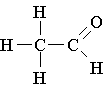
E. It has a higher boiling point than an alcohol of similar molecular weight
11. Alkanes and alkenes are similar in all the following properties EXCEPT
A. solubility in non-polar solvents
B. insolubility in water
C. flamability
D. reactivity
E. lack of toxicity
12. The symbol [O] written above a reaction arrow means
A. oxygen is removed from one of the reactants during the reaction
B. the reaction consumes oxygen from the atmosphere
C. that an oxidation is occurring
D. that a reduction reaction is occurring and oxygen is liberated
E. None of the above
13. Which compound is an example of an amine salt?
A. 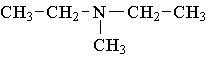 B.
B. 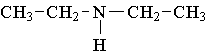 C.
C. 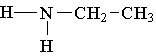
D. 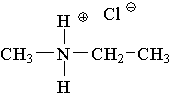 E.
E. 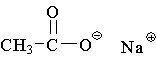
14. Pick the best statement
A. The two optical isomers of a chiral bio molecule behave differently because enzyme active sites are generally chiral
B. cis- and trans- isomers are not structural isomers because the connections of the atoms are the same
C. structural isomers have the same molecular formula but different connections
D. AlaPhe and PheAla are examples of structural isomers
E. All of these are true
15. Which of the following is an example of a secondary amine?
A. 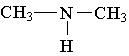 B.
B. 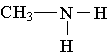 C.
C. 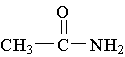
D.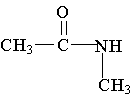 E.
E. 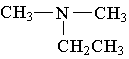
16. Which molecule is not an isomer of the molecule C2H5-O-C2H5 ?
A. CH3-O-CH2CH2CH3
B. C4H9OH
C. CH3CH2CH(OH)CH3
D. CH3CH(CH3)CH2OH
E. CH3CH2COCH3
17. The cause of cis-trans isomerism is
A. stability of the double bond
B. lack of free rotation along the double bond
C. strength of the double bond
D. short length of the double bond
E. vibration of the double bond
18. The amino acid sequence of a protein is known as its ________________
A. primary structure
B. secondary structure
C. tertiary structure
D. quaternary structure
E. None of the above
19. In the tetrapeptide AlaCysValLeu, the C-terminal amino acid is ____
and the N-terminal amino acid is ____.
A. Ala, Leu B. Cys, Val C. Leu, Ala D. Val, Cys E. None of these
20. All of the following are non-covalent interactions important in maintaining the secondary, tertiary, and quaternary aspects of a protein EXCEPT
A. disulfide bridges
B. hydrogen bonding within the backbone
C. hydrogen bonding between side groups (we have been labeling them G )
D. salt bridges
E. hydrophobic interactions
21. Proteins which consist of two or more chains assembled into a 3-dimensional structure are said to display
A. primary structure
B. secondary structure
C. tertiary structure
D. quaternary structure
E. None of the above
22. When a protein is ____, its primary structure is destroyed, thus destroying the other aspects of its structure.
A. denatured B. hydrolyzed C. ionized D. polymerized E. esterified
23. All the following can denature proteins without hydrolysis EXCEPT
A. heat B. mechanical stress C. enzyme treatment D. lowering the pH E. heavy metal ions
24. Enzymes are members of which class of bio molecules?
A. carbohydrates B. lipids C. nucleic acids D. proteins E. steroids
25. All the following phrases correctly describe enzymes EXCEPT
A. dissolve in water B. have a globular shape C. behave as substrates
D. contain an active site E. act as catalysts
26. Which factor is NOT important in explaining how enzymes work?
A. Two different substrate molecules are brought into close contact
B. Substrates are brought into solution more easily
C. The bonds in substrates are subjected to strains which weaken them
D. Substrates are forced into the correct orientation for interaction
E. Substrates are placed near acidic or basic sites
27. Which of the following is a major function of nucleic acids?
A. storage and transfer of genetic information
B. storage and intracellular transfer of energy
C. catalysis of virtually all biochemical reactions
D. structural support in both plants and animals
E. long term storage of energy
28. The backbone of a nucleic acid molecule consists of
A. alternating sugar and phosphate groups linked by phosphate ester bonds.
B. alternating sugar and nitrogen base groups linked by amide bonds
C. alternating nitrogen bases and phosphate groups linked by amide bonds
D. sugar molecules bonded from the #3 carbon of one molecule to the #5 carbon of the other by glycosidic linkages
E. complementary bases joined by hydrogen bonds
29. In DNA, a DNA sequence complementary to C-G-G-T-T-A-G is
A. G-C-C-A-A-T-C
B. C-G-G-T-T-A-G
C. A-T-T-G-G-C-T
D. G-C-C-U-U-U-C
E. G-C-C-U-U-A-C
30. The process in which information from DNA is used to manufacture RNA is called
A. mutation B. replication C. transcription D. translation E. translocation
31. All of the following statements about RNA are true EXCEPT
A. RNA can exist in three forms: rRNA, tRNA, and mRNA
B. RNA molecules are smaller than DNA molecules and form double helices like DNA
C. RNA does not contain thymine (T)
D. Transfer RNA delivers amino acids to the protein chain as it is being manufactured
E. Messenger RNA is complementary to the template strand of its DNA and contains the essential sequence information from the gene
32. Pick the most accurate statement.
A. Plants and mammals both contain DNA, but plants use carbohydrates instead of proteins.
B. All living systems contain proteins, but plants and animals do not use the same set of amino acids.
C. Nucleic acids and proteins are built from the same small set of amino acids.
D. DNA is taken apart nucleotide by nucleotide to build the proteins a living system requires.
E. Nucleic acids and proteins have similar structures in that both consist of an unchanging, repeating backbone.
33. One of the main reasons for using recombinant DNA techniques is
A. circular DNA loops are more symmetrical than the double helix.
B. to improve upon the accuracy of building the desired proteins
C. to improve upon the speed of building the desired proteins.
D. to create DNA without genetic defects.
E. to create DNA that is better at dealing with radiation damage.
34. Place in order of decreasing molecular weight: a. tRNA, b. gene c. mRNA d. DNA e. vitamin
A. a > b > c > d > e B. d > b > c > a > e C. e > d > c > b > a D. d > b > a > c > e
35. All of the following are functions of proteins EXCEPT
A. transport of necessary chemicals
B. protection against foreign substances
C. movement
D. control of biochemical reactions
E. storage of energy
36. Which of the following statements regarding the Amylases and Starch experiment is the most accurate description of the experiment?
A. The blue color is used to tell when the reaction solution is still basic (alkaline).
B. The breakdown of starch is occurring in the dropper being held in the fingers.
C. The breakdown of starch occurs in the multi-well tray.
D. The amylase creates starch from sugar in the dropper being held in the fingers.
E. The amylase creates starch from sugar in the multi-well tray.
37. Describe and give the names of four (4) distinctly different types of polymers we have studied. Provide enough detail to show that you understand the basic structures of the polymers. You may describe the polymerization process that creates each polymer if that helps you describe the structure. Hint: if you look back over this exam, at least 5 different polymer types are mentioned.
2.5 pts
2.5 pts
2.5 pts
2.5 pts
Multiple choice answers: put the correct letter in each box
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
# correct__________×2.5 = Total Exam Points ____________