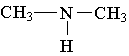
 B.
B. 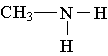 C.
C. 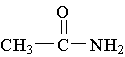
Exam 2
CH 130
May, 2000
There are 5 pages and 26 questions on this exam. Be sure you have them all.
Write your answers to the multiple choice questions in the numbered boxes on the last page of this exam. Each of the 24 multiple choice problems are worth 3.8 points.
1. All of the following are properties of amines EXCEPT
A. They frequently have offensive odors.
B. Those that can form hydrogen bonds have higher boiling points than expected for their molecular weight.
C. Those with low molecular weight are soluble in water.
D. They react with acids to form amides.
E. They act as bases in many reactions.
2. All of the following statements concerning the carbonyl group in aldehydes and ketones are true EXCEPT
A. The bond is polar, with a slight negative charge on the oxygen atom.
B. The bond angles about the central carbon are 120.
C. The carbonyl group is planar.
D. In condensed form the carbonyl group can be written as -CHO or -CO-.
E. Because the bond is polar, carbonyl groups readily form hydrogen bonds with each other.
3. Which of the following is an example of a primary amine?
A.  B.
B. 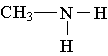 C.
C. 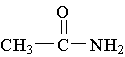
D.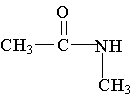 E.
E. 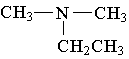
4. What is the product of the reduction of 3-methyl-2-pentanone?
A. 3-methyl-2-pentanol
B. 2-methyl-3-pentanol
C. 3-methyl-2-pentanal
D. 3-methyl-2-pentene
E. no reaction
5. A compound with an -OH group and an ether-like -OR group bonded to the same carbon atom is a(an)
A. acetal B. aldol C. diol D. hemiacetal E. simple ether
6. If methylamine reacts with hydrochloric acid, the major product will be
A. ammonium chloride
B. dimethylammonium chloride
C. methylammonium chloride
D. trimethylammonium chloride
E. methylammonium hydroxide
7. The products of basic hydrolysis of an ester are
A. another ester and water
B. alcohol and carboxylic acid
C. alcohol and water
D. carboxylic acid and water
E. carboxylate ion and alcohol
8. All of the following statements about carboxylic acids are true EXCEPT
A. They undergo substitution reactions involving the -OH group
B. At low molecular weights they are liquids with sharp stinging odors
C. They form hydrogen bonds, causing their boiling points to be higher than expected on the basis of molecular weight
D. They react with bases to form salts which are often more soluble than the original acid
E. When they behave as acids, the -OH group is lost leaving the CO- ion
9. Which molecule is a ketone?
A. 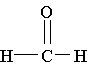
B. 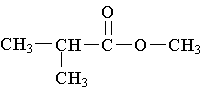
C.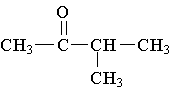
D.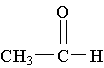 E.
E.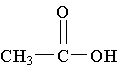
10. Which equation best represents the dissociation of a carboxylic acid in water?
A. CH3COOH + H2O CH3COOH2+ + OH-
B. CH3COOH CH3COO- + H+
C. CH3COOH + H2O CH3COO- + H3O+
D. CH3COOH + H3O+ CH3COOH2+ + H2O
E. CH3COOH + 2 H2O CH3COO- + 2 H3O+
11. Which structure represents a zwitterion?
A.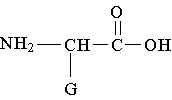 B.
B. 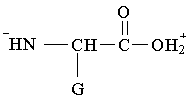 C.
C. 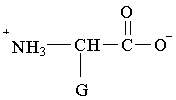
D. 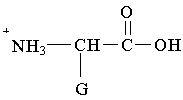 E.
E. 
12. Which pair of compounds can react to form a hemiacetal?
A. CH3CH2CHO and CH3CH2OH
B. CH3COCH3 and CH3CH2CHO
C. CH3CH2CHO and CH3COOH
D. CH3COCH3 and CH3COOH
E. CH3COOH and CH3CH2OH
13. When an amine behaves as a base it ______ a hydrogen ion to form a(an) _______ ion.
A. loses ; hydronium
B. loses ; ammonium
C. loses ; hydroxide
D. gains ; ammonium
E. gains ; hydronium
14. Which carboxylic acid is used to prepare the ester
shown? 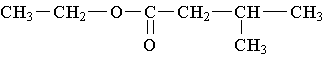
A. CH3-CH2-COOH B. CH3-COOH
C. (CH3)2-CH-COOH D. (CH3)2-CH-CH2-COOH E. CH3-(CH2)3-COOH
15. Which of the following statements about Nylon is true?
A. Nylon is a polyester
B. Nylon will not dissolve in strong acid or base
C. Nylon is the product of the reaction between a dicarboxylic acid and a dialcohol
D. Nylon is the product of the reaction between a dicarboxylic acid and a diamine
E. The monomers in Nylon are bonded together by hydrogen bonds
16. The peptide bond joining amino acids into proteins is a specific example of the _____ bond.
A. amide B. carbonyl C. ester D. glycosidic E. hydrogen
17. Two functional groups that are present in all amino acids are the _____ group and the _____ group.
A. hydroxyl ; amide
B. carboxyl ; amine
C. carboxyl ; phosphate ester
D. acetal ; amine
E. carbonyl ; amide
18. Which molecule is an alpha amino acid?
A. 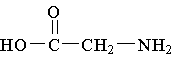 B.
B. 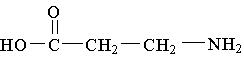 C.
C. 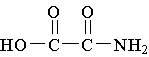
D. 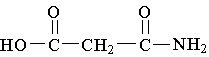 E.
E. 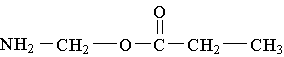
19. Reaction of an ester with a strong base is called
A. reverse esterification
B. esterification
C. saponification
D. oxidation
E. condensation
20. What is the common name of the
molecule shown?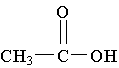
A. acetic acid
B. ascorbic acid
C. formic acid
D. lactic acid
E. oxalic acid
21. In lecture we discussed the molecule shown to the right, whose optical isomers are responsible for the taste and odor of caraway and spearmint. Which carbon is chiral?
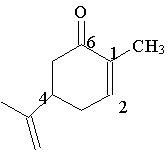
A. 1
B. 2
C. 4
D. 6
E. none of these choices
22. Which of the following statements about soap is NOT true?
A. When fat is saponified, the products are soap and glycerol
B. The polar carboxylate end of the soap molecule makes it soluble in water
C. The non-polar tail of the soap molecule is attracted to oily dirt
D. Soap is produced when fatty acids react with glycerol
E. Detergent is designed to work better than soap in hard water
23. Regarding the lessons of the Acid Deposition
experiment, which of the following is NOT true?
A. Certain acids can move through the atmosphere by gaseous diffusion
B. The "eye" forms in the blue indicator drop when acid is deposited in the outer surface of the drop
C. The product of the gaseous reaction between HCl and NH3 is a solid
D. Marble statues are corroded by basic raindrops
E. Acid rain is dropping the pH of lakes and natural waters
24. Which of the following statements is NOT true?
A. A chiral molecule is superimposable on its mirror image
B. Optical isomers of each other have the same solubility in water
C. Optical isomers will generally react differently with a chiral molecule
D. All but one natural amino acids are chiral
E. All natural proteins are chiral
25. Label the lowest boiling amine in the following set.
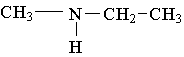
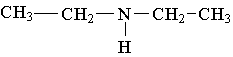
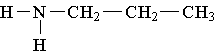
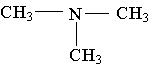
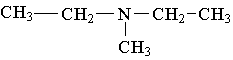
26. Write the family name (such as carboxylic acid) beneath each of the following
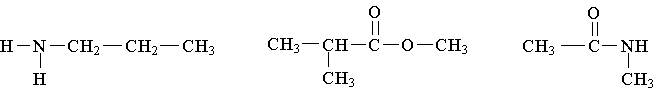
Multiple choice answers: put the correct letter in each box
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
# Multiple.Ch.______×3.8 = ______ Points from 25+26 _______ Total Exam Points ________