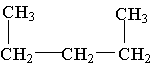
Exam 1
CH 130
April, 2000
There are 5 pages and 28 questions on this exam. Be sure you have them all.
Write your answers to the multiple choice questions in the numbered boxes on the last page of this exam. Each of the 24 multiple choice problems are worth 3 points.
1. Which of the following statements about the behavior of the element carbon in organic compounds is incorrect?
A. Carbon can be involved in polar covalent bonds.
B. Carbon can form single, double, or triple bonds with other carbon atoms.
C. Carbon always forms four bonds.
D. The bond angles around carbon are 90o.
E. In addition to other carbon atoms, carbon is likely to form bonds with hydrogen, nitrogen, or oxygen.
2. Two or more compounds with the same molecular formula but with the atoms connected differently are referred to as
A. normal alkanes
B. branched alkanes
C. functional groups
D. isomers
E. conformations
3. All the choices listed are representations of the same molecule except
A. C5H12
B. CH3CH2CH2CH2CH3
C. 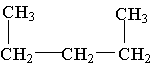
D.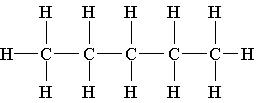
E. (CH3)2 CH2 CH2 CH3
4. The molecule shown is named as a substituted _________ because _________ .
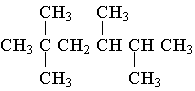
A. decane; it contains 10 atoms of carbon.
B. hexane; it contains six atoms of carbon in its longest chain.
C. tetramethane; it contains four methyl groups as branches.
D. hexamethane; it contains six methyl groups all together.
E. butane; four carbons are substituted onto the chain.
5. In the molecule 3,3-dimethylhexane, carbon number 4 is
A. primary B. secondary C. tertiary D. quaternary E. none of these
6. Which molecule is an isomer of:

A. 
B. 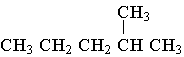
C. 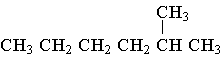
D. 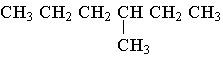
E. ![]()
7. What is the IUPAC name of the compound shown:

A. 2-ethyl-3,5-dimethylhexane
B. 3,5-dimethyl-2-ethylhexane
C. 2,4,5-trimethylheptane
D. 3,4,6-trimethylheptane
E. 5-ethyl-2,4-dimethylhexane
8. How many hydrogens are present in the molecule shown:
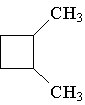
A. 6 B. 18 C. 14 D. 16 E. 12
9. In organic chemistry, the term unsaturated means a molecule
A. which has the maximum number of carbon-hydrogen bonds possible
B. with a specific six-membered ring structure
C. which contains one or more multiple bonds between carbon atoms
D. which can react by taking up one or more water molecules
E. which is formed from many smaller molecules
10. What is the IUPAC name of the molecule shown:
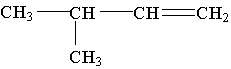
A. isopentene
B. 3-methyl-1-butene
C. 3-methyl-1,2-butene
D. 2-methyl-3-butene
E. 1,1-dimethyl-2-propene
11. Which molecule can have cis-trans isomers?
A. CH3CH=C(CH3)2
B. (CH3)2C=CHCH3
C. (CH3)2C=C(CH3)2
D. CH3CH=CHCH3
E. CH3CH=CCl2
12. What is the IUPAC name of the molecule shown:
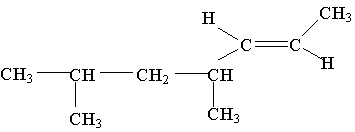
A. trans-4,6-dimethyl-2-heptene
B. cis-4,6-dimethyl-2-heptene
C. trans-2-nonene
D. cis-2-nonene
E. trans-2,4-dimethyl-5-heptene
13. All the following are properties of alkenes except
A. soluble in non-polar organic solvents
B. low boiling points
C. flammable
D. less reactive than corresponding alkanes
E. may exist as cis-trans isomers
14. Chemical reactions involving carbon-carbon double bonds are generally referred to as __________ reactions.
A. substitution B. addition C. oxidation D. reduction E. combustion
15. When 2-butene reacts with Br2, the product is
A. 2-bromobutane B. 3-bromobutane C. 1,2-dibromobutane
D. 1,3-dibromobutane E. 2,3-dibromobutane
16. The concept that explains why aromatic compounds resist attack to the ring is
A. double bonding B. cis-trans isomerism C. resonance D. polymerization E. oxidation
17. Which of the following organic compounds are most likely to form hydrogen bonds:
A. alkanes B. alkenes C. alcohols D. aromatics E. ethers
18. The molecule which has three carbon atoms with an OH group on each and is used as a moisturizer is
A. ethanol B. glycerol C. glycol D. methanol E. phenol
19. What is the IUPAC name of the following compound:
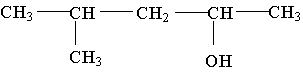
A. 4-methyl-2-pentanol
B. 2-methyl-4-pentanol
C. 4,4-dimethyl-2-butanol
D. 2,2-dimethyl-4-butanol
E. 2-isohexanol
20. How many carbons are in the molecule represented by
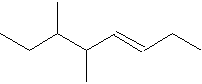
A. 6 B. 8 C. 10 D. 12 E. 20
21. The molecule shown is a ______ alcohol because _________.
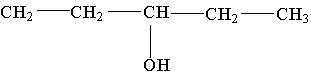
A. primary; it has one OH group
B. primary; its OH group is on the end of the carbon chain
C. secondary; the carbon bonded to the OH group is bonded to two other carbons
D. secondary; there are two carbons on either side of the carbon containing the OH group
E. tertiary; the OH group is bonded to the number 3 carbon
22. What is the IUPAC name of the following compound: CH3 - CH2- CH2 - O - CH3
A. 1,2-etherbutane B. methylpropyl ether C. ethylmethyl ether D. butyl ether E. isobutyl ether
23. The hybridization of the carbons in diamond is _____ and in graphite it is _______.
A sp2; sp3 B. sp3; sp2 C. sp3; sp D. sp; sp3 E. sp; sp2
24. The following question refers to the laboratory experiment on "60 mg a Day." Suppose 2 drops of grapefruit juice require 10 drops of DCP whereas 2 drops of standard 0.50 mg/mL vitamin-C solution require 5 drops of DCP. What is the concentration of vitamin-C in the grapefruit juice in mg/mL?
A. 1.0 mg/mL B. 0.5 mg/mL C. 0.25 mg/mL D. 1.6 mg/mL E. none of these
25. Label the molecule that is most soluble in water and label the molecule that is least soluble in water. Briefly justify your reasoning.
CH3 - CH2 - CH2 - CH2 - OH
CH3 - CH2 - CH2 - OH
CH3 - CH2 - CH2 - CH3
CH3 - CH2 - CH3
CH3 - CH2 -CH2 - CH2 - CH2 - CH2 - CH2 - CH3
26. Label the molecule that has the highest boiling point and label the molecule that has the lowest boiling point. Briefly justify your reasoning.
CH3 - CH2- CH2 - OH
CH3 - CH2 - CH2 - CH2 - OH
CH3 - CH2 - O - CH2 - CH3
CH3 - CH2 - O - CH3
CH3 - CH2 - CH2- O - CH3
27. What polymer would result from polymerizing the compound CH2=CHCH2CH3? Draw enough of the polymer to be clear in your answer.
28. Draw one real example (an actual molecule) of each of the following and show the product(s), if any, that would be produced if each of your compounds were mildly oxidized:
primary alcohol secondary alcohol tertiary alcohol
Multiple choice answers: put the correct letter in each box
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |