A vitamin is an organic compound required as a nutrient in tiny amounts by an organism.[1] A compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on the circumstances and the particular organism. For example, ascorbic acid functions as vitamin C for some animals but not others, and vitamins D and K are required in the human diet only in certain circumstances.[2] The term vitamin does not include other essential nutrients such as dietary minerals, essential fatty acids, or essential amino acids, nor does it encompass the large number of other nutrients that promote health but are otherwise required less often.[3] Vitamins are classified by their biological and chemical activity, not their structure. Thus, each "vitamin" may refer to several vitamer compounds that all show the biological activity associated with a particular vitamin. Such a set of chemicals are grouped under an alphabetized vitamin "generic descriptor" title, such as "vitamin A," which includes the compounds retinal, retinol, and many carotenoids.[4] Vitamers are often inter-converted in the body. Vitamins have diverse biochemical functions, including function as hormones (e.g. vitamin D), antioxidants (e.g. vitamin E), and mediators of cell signaling and regulators of cell and tissue growth and differentiation (e.g. vitamin A).[5] The largest number of vitamins (e.g. B complex vitamins) function as precursors for enzyme cofactor bio-molecules (coenzymes), that help act as catalysts and substrates in metabolism. When acting as part of a catalyst, vitamins are bound to enzymes and are called prosthetic groups. For example, biotin is part of enzymes involved in making fatty acids. Vitamins also act as coenzymes to carry chemical groups between enzymes. For example, folic acid carries various forms of carbon group – methyl, formyl and methylene - in the cell. Although these roles in assisting enzyme reactions are vitamins' best-known function, the other vitamin functions are equally important.[6] Until the 1900s, vitamins were obtained solely through food intake, and changes in diet (which, for example, could occur during a particular growing season) can alter the types and amounts of vitamins ingested. Vitamins have been produced as commodity chemicals and made widely available as inexpensive pills for several decades,[7] allowing supplementation of the dietary intake.
Vitamer chemical name(s) (list not complete) ![]()
Recommended dietary allowances
(male, age 19–70)[18]
Deficiency disease
Upper Intake Level
(UL/day)[18]
Overdose disease
Retinoids
(retinol,
retinoids
and
carotenoids)
Fat
900 µg
3,000 µg
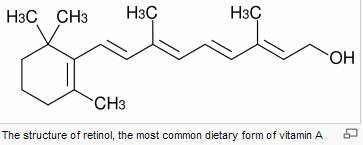
Vitamin A plays a role in a variety of functions throughout the body, such as:
- Vision
- Gene transcription
- Immune function
- Embryonic development and reproduction
- Bone metabolism
- Haematopoiesis
- Skin health
- Reducing risk of heart disease
- Antioxidant Activity
Water
1.2 mg
N/D[20]
Rare hypersensitive reactions resembling anaphylactic shock—injection only;
Drowsiness
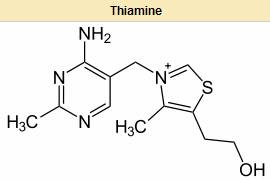
the catabolism of sugars and amino acids
Water
1.3 mg
N/D
?
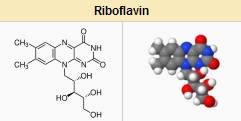
Function and Mechanism of Action FMN and FAD function as coenzymes for a wide variety of oxidative enzymes and remain bound to the enzymes during the oxidation-reduction reactions. Flavins can act as oxidizing agents because of their ability to accept a pair of hydrogen atoms. Reduction of isoalloxazine ring (FAD, FMN oxidized form) yields the reduced forms of the flavoproteins (FMNH2 and FADH2)(5). Flavoproteins exhibit a wide range of redox potential and therefore can play a wide variety of roles in intermediary metabolism (5). Some of these roles are:
Flavoproteins play very important roles in the electron transport chain(5)
Decarboxylation of pyruvate and α-Ketoglutarate requires FAD()
Fatty acyl CoA dehydrogenase requires FAD in fatty acid oxidation (5)
FAD is required to the production of pyridoxic acid from pyridoxal (vitamin B6)
The primary coenzyme form of vitamin B6 (Pyridoxal phosphate) is FMN dependent(5)
FAD is required to convert retinal (Vitamin A) to retinoic acid
Synthesis of an active form of folate (5-methyl THF) is FADH2 dependent
FAD is required to convert tryptophan to niacin (vitamin B3)
Reduction of the oxidized form of glutathione (GSSG) to its reduced form (GSH) is also FAD dependent (5)
Riboflavin has been used in several clinical and therapeutic situations. For over 30 years, riboflavin supplements have been used as part of the phototherapy treatment of neonatal jaundice. The light used to irradiate the infants breaks down not only the toxin causing the jaundice, but the naturally occurring riboflavin within the infant's blood as well.
More recently there has been growing evidence that supplemental riboflavin may be a useful additive along with beta-blockers in the prevention of migraine headaches.[20]
Development is underway to use riboflavin to improve the safety of transfused blood by reducing pathogens found in collected blood. Riboflavin attaches itself to the nucleic acids (DNA and RNA) in cells, and when light is applied, the nucleic acids are broken, effectively killing those cells. The technology has been shown to be effective for inactivating pathogens in all three major blood components: (platelets, red blood cells, and plasma). It has been shown to inactivate a broad spectrum of pathogens, including known and emerging viruses, bacteria, and parasites.
Recently riboflavin has been used in a new treatment to slow or stop the progression of the corneal disorder keratoconus. This is called corneal collagen crosslinking (CXL). In corneal crosslinking, riboflavin drops are applied to the patient’s corneal surface. Once the riboflavin has penetrated through the cornea, Ultraviolet A light therapy is applied. This induces collagen crosslinking, which increases the tensile strength of the cornea. The treatment has been shown in several studies to stabilize keratoconus.
Water
16.0 mg
35.0 mg
Liver damage (doses > 2g/day)[21] and other problems
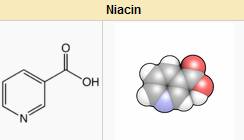
Niacin, also known as vitamin B3 or nicotinic acid, is a water-soluble vitamin that prevents the deficiency disease pellagra. It is an organic compound with the molecular formula C6H5NO2. It is a derivative of pyridine, with a carboxyl group (COOH) at the 3-position. Other forms of vitamin B3 include the corresponding amide, nicotinamide ("niacinamide"), where the carboxyl group has been replaced by a carboxamide group (CONH2), as well as more complex amides and a variety of esters. The terms niacin, nicotinamide, and vitamin B3 are often used interchangeably to refer to any one of this family of molecules, since they have a common biochemical activity.
Niacin is converted to nicotinamide and then to NAD and NADP in vivo. Although the two are identical in their vitamin activity, nicotinamide does not have the same pharmacological effects as niacin, which occur as side-effects of niacin's conversion. Thus nicotinamide does not reduce cholesterol or cause flushing,[1] although nicotinamide may be toxic to the liver at doses exceeding 3 g/day for adults.[2] Niacin is a precursor to NADH, NAD, NAD+, NADP and NADPH, which play essential metabolic roles in living cells.[3] Niacin is involved in both DNA repair, and the production of steroid hormones in the adrenal gland.
Niacin is one of five vitamins associated with a pandemic deficiency disease: these are niacin (pellagra), vitamin C (scurvy), thiamin (beriberi), vitamin D (rickets), and vitamin A (vitamin A deficiency, which has no common name but is one of the most common symptomatic deficiencies worldwide).
Water
5.0 mg[22]
N/D
?
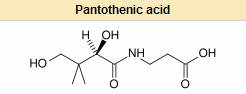
Pantothenic acid, also called vitamin B5 (a B vitamin), is a water-soluble vitamin required to sustain life (essential nutrient). Pantothenic acid is needed to form coenzyme-A (CoA), and is critical in the metabolism and synthesis of carbohydrates, proteins, and fats. In chemical structure, it is the amide between D-pantoate and beta-alanine. Its name is derived from the Greek pantothen (παντόθεν) meaning "from everywhere" and small quantities of pantothenic acid are found in nearly every food, with high amounts in whole-grain cereals, legumes, eggs, meat, and royal jelly. It is commonly found as its alcohol analog, the provitamin panthenol, and as calcium pantothenate.
Only the dextrorotatory (D) isomer of pantothenic acid possesses biologic activity.[1] The levorotatory (L) form may antagonize the effects of the dextrorotatory isomer.[2]
Water
1.3–1.7 mg
100 mg
Impairment of proprioception, nerve damage (doses > 100 mg/day)
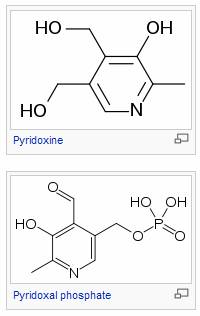
Vitamin B6 is a water-soluble vitamin and is part of the vitamin B complex group. Pyridoxal phosphate (PLP) is the active form and is a cofactor in many reactions of amino acid metabolism, including transamination, deamination, and decarboxylation. PLP also is necessary for the enzymatic reaction governing the release of glucose from glycogen.
Water
30.0 µg
N/D
?
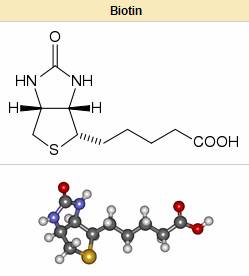
Biotin is necessary for cell growth, the production of fatty acids, and the metabolism of fats and amino acids. It plays a role in the Citric acid cycle, which is the process by which biochemical energy is generated during aerobic respiration. Biotin not only assists in various metabolic reactions, but also helps to transfer carbon dioxide. Biotin is also helpful in maintaining a steady blood sugar level. Biotin is often recommended for strengthening hair and nails. Consequently, it is found in many cosmetic and health products for the hair and skin.
Deficiency is extremely rare, as intestinal bacteria generally produce an excess of the body's daily requirement. For that reason, statutory agencies in many countries (e.g., the Australian Department of Health and Aging) do not prescribe a recommended daily intake.
Water
400 µg
Deficiency during pregnancy is associated with birth defects, such as neural tube defects
1,000 µg
Possible decrease in seizure threshold
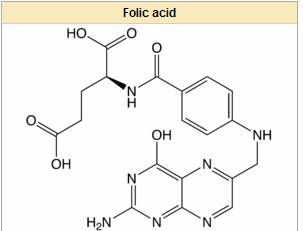
amino acid production
Water
2.4 µg
N/D
No known toxicity[25]
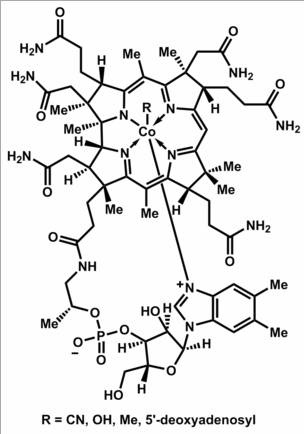
Vitamin B12 is a water soluble vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood. It is one of the eight B vitamins. It is normally involved in the metabolism of every cell of the body, especially affecting DNA synthesis and regulation, but also fatty acid synthesis and energy production.
Water
90.0 mg
2,000 mg
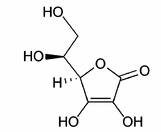
Vitamin C or L-ascorbic acid is an essential nutrient for humans, a large number of higher primate species, a small number of other mammalian species (notably guinea pigs and bats), a few species of birds, and some fish.[1]
Ascorbate (an ion of ascorbic acid) is required for a range of essential metabolic reactions in all animals and plants. It is made internally by almost all organisms, humans being a notable exception. Deficiency in this vitamin causes scurvy in humans.[2][3][4] It is also widely used as a food additive.
Fat
5.0 µg–10 µg[26]
Rickets and Osteomalacia
50 µg
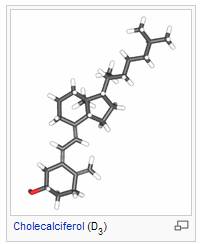
Vitamin D is a group of fat-soluble prohormones, the two major forms of which are vitamin D2 (or ergocalciferol) and vitamin D3 (or cholecalciferol).[1] The term vitamin D also refers to metabolites and other analogues of these substances. Vitamin D3 is produced in skin exposed to sunlight, specifically ultraviolet B radiation.
Vitamin D plays an important role in the maintenance of organ systems.[2]
Vitamin D regulates the calcium and phosphorus levels in the blood by promoting their absorption from food in the intestines, and by promoting re-absorption of calcium in the kidneys, which enables normal mineralization of bone and prevents hypocalcemic tetany. It is also needed for bone growth and bone remodeling by osteoblasts and osteoclasts.[3][4].
In the absence of vitamin K or with drugs (particularly blood thinners) that interfere with Vitamin K metabolism, Vitamin D can promote soft tissue calcification.[5]
It inhibits parathyroid hormone secretion from the parathyroid gland.[6][7]
Vitamin D affects the immune system by promoting phagocytosis, anti-tumor activity, and immunomodulatory functions. [8]
Fat
15.0 mg
Deficiency is very rare; mild hemolytic anemia in newborn infants.[27]
1,000 mg
Increased congestive heart failure seen in one large randomized study.[28]
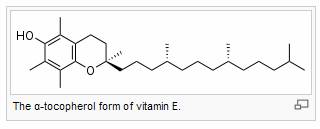
Vitamin E is the collective name for a set of 8 related α-, β-, γ-, and δ-tocopherols and the corresponding four tocotrienols, which are fat-soluble vitamins with antioxidant properties.[1][2] Of these, α-tocopherol (also written as alpha-tocopherol) has been most studied as it has the highest bioavailability.[3]
It has been claimed that α-tocopherol is the most important lipid-soluble antioxidant, and that it protects cell membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.[1][4] This would remove the free radical intermediates and prevent the oxidation reaction from continuing. The oxidised α-tocopheroxyl radicals produced in this process may be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol or ubiquinol.[5] However, the importance of the antioxidant properties of this molecule at the concentrations present in the body are not clear and it is possible that the reason why vitamin E is required in the diet is unrelated to its ability to act as an antioxidant.[6]. Other forms of vitamin E have their own unique properties. For example, γ-tocopherol (also written as gamma-tocopherol) is a nucleophile that may react with electrophilic mutagens[3]; and the tocotrienols having specialized roles in protecting neurons from damage[7], cancer prevention[8] and cholesterol reduction[9] by inhibiting the activity of HMG-CoA reductase[16-1];δ-tocotrienol blocks processing of sterol regulatory element‐binding proteins (SREBPs)[16-1].However, the roles and importance of all of the various forms of vitamin E are presently unclear,[10][11] and it has even been suggested that the most important function of vitamin E is as a signaling molecule, and that it has no significant role in antioxidant metabolism.[12][13]
Most studies about vitamin E have supplemented using only the synthetic alpha-tocopherol, but doing so leads to reduced serum gamma- and delta-tocopherol concentrations. Moreover, a 2007 clinical study involving synthetic alpha-tocopherol concluded that supplementation did not reduce the risk of major cardiovascular events in middle aged and older men[14]. For more info, read article tocopherol.
Fat
120 µg
N/D

Vitamin K is involved in the carboxylation of certain glutamate residues in proteins to form gamma-carboxyglutamate residues (abbreviated Gla-residues). The modified residues are often (but not always) situated within specific protein domains called Gla domains. Gla-residues are usually involved in binding calcium. The Gla-residues are essential for the biological activity of all known Gla-proteins.[5]
At this time[update] 14 human proteins with Gla domains have been discovered, and they play key roles in the regulation of three physiological processes:
Blood coagulation: (prothrombin (factor II), factors VII, IX, X, protein C, protein S and protein Z).[6]
Bone metabolism: osteocalcin, also called bone Gla-protein (BGP), and matrix gla protein (MGP).[7]
Vascular biology.[8]