Experimental Guideline: Titration of butyllithium
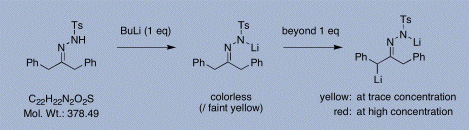
Butyllithium (and other stock alkyllithiums) must be titrated regularly. Many methods are available for this purpose; however, one of the most reliable procedures employs 1,3-diphenylacetone p-tosylhydrazone (see Shapiro et al. J. Organomet. Chem. 1980, 186 (2), 155-158). This crystalline compound is non-hygroscopic, easily and accurately dispensed (helped by a high MW of 378.5), and its dianion has a red coloration (at high concentration): the end-point of the titration (equivalence point) is the first appearance of a deep yellow color indicative of a persistent low concentration of dianion (NOT to orange/red). The notes and photographs below illustrate the method.




end-point: persistent (deep) yellow at 0.52 mL of added BuLi (titer = 0.769/0.52 = 1.48 M)

