Interaction of a solvent has profound influences that are often overlooked. For example, the simple act of dissolving a molecule may provide the energetic change needed for SN1 ionization to occur. Also, changing solvents from polar to nonpolar (or vice versa) can change the rate constant by many orders of magnitude.
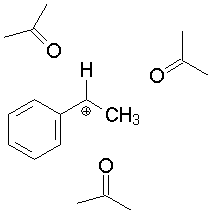
Look at an example of a carbocation surrounded by acetone solvent molecules:
Solvation shell around the 1-phenylethyl cation (acetone
is the solvent in this example). |
In this case, the dipole of the acetone molecules tends to orient itself to stabilize the positive charge and disperse it.
Another impact of solvation is that the solvent shell has to reorganize itself as ionization occurs. The solvent molecule on the side of the leaving group has to "get out of the way" as the leaving group departs. This leads to the opportunity for solvent (or other external nucleophiles) to react at any of several distinct stages:
| No ionization | Contact or tight ion pair | Solvent-separated ion pair | Free ions |
| Sn2.pdb | TIP.pdb | SSIP.pdb | freeions.pdb |
Consequences:
|
Consequences:
|
Consequences:
|
Consequences:
|
| Example: Goering, H. L; Towns, D. L.; Dittmar, B. J. Org. Chem., 1962, 27, 736-739. | Example: Diaz, A. F.; Lazdins, I;, Winstein, S. J. Am. Chem. Soc., 1968, 90, 1904-5. | Example:Richard, J. P.; Amyes, T. L; Vontor, T. J. Am. Chem. Soc., 1991, 113, 5871-3 (electron-withdrawing substituents). | Example: Richard, J. P.; Amyes, T. L; Vontor, T. J. Am. Chem. Soc., 1991, 113, 5871-3 (electron-donating substituents). |
Back to CH 630 Home page
Last updated: 09/21/2000