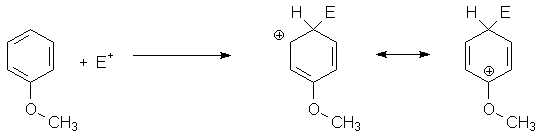
Although oxygen is an electronegative element, it can share its lone pair electrons to stabilize positive charges on carbon. This is seen in resonance stabilization in p-complexes formed in electrophilic aromatic substitution:

Compare the electrostatic potential maps (scaled identically) for reaction of benzene (top) and methoxybenzene (bottom) with a proton. The neutral compound is on the left, the protonated intermediate on the right:
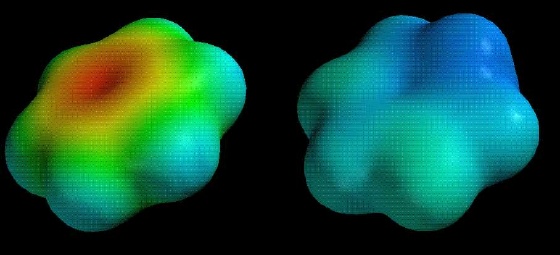
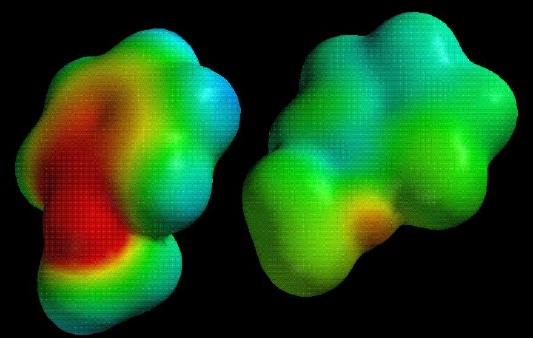
Notice how much less positive (blue) the methoxybenzene intermediate is!
Back to CH630 Home Page
Last updated: 09/25/03
Comments to K. Gable