Removal of a weakly acidic proton from a carbonyl or enol leads to a resonance-stabilized carbanion. Normally, if a lithiated base (e.g., LDA) is used, the enolate that is formed will have the lithium coordinated to oxygen, and aggregation will occur (see Carey & Sundberg, p. 427).
In assessing reactivity through the use of conformational analysis, one must use the double-bonded nature of the enolate. This is obvious when considering the structure of, for example, cyclohexanone enaolate:
| CHenolate.pdb |
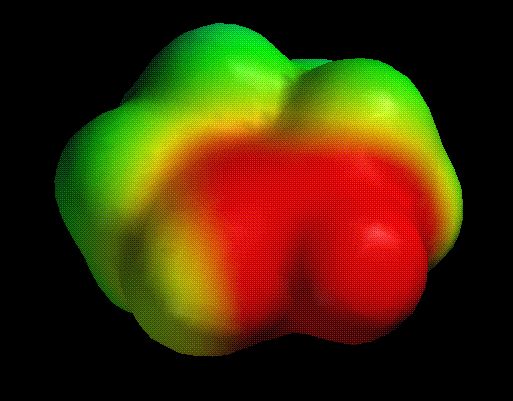
The delocalized nature of the enolate (and the potential for reaction at either C or O)
is revealed by the electrostatic potential map:
|
Aggregation is as important for enolates as it is for alkyllithium compounds:
| Adapted from Elliard & Carpenter, J. Am. Chem. Soc., 1985, 107, 3345. | |
| Adapted from Amstutz, Schweizer, Seebach and Dunitz, Helv. Chem. Acta, 1981, 64, 2617. |
As with alkyllithium reagents, chelators and strongly coordinating solvents can break
up aggregates and help control the steric environment of the reagent during nucleophilic
attack. A recent paper
was published concerning the reactivity of monomers vs. dimers.
(Subscription or access from the OSU campus required; you will also need the Acrobat
reader plugin)
Enamines are closely related to enols. They have a similar delocalized structure, but are more polarizable and lack the negative charge (and basicity) of the enolate. Compare the structure and electrostatic potential of an enamine made from cyclohexanone:
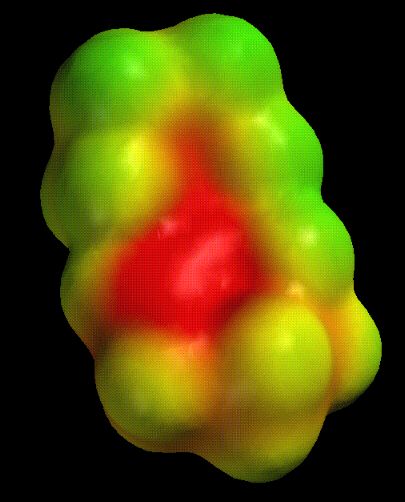 |
|
| enamine.pdb |
One important difference is that the choice of the amine used can have a dramatic effect on the stereoselectivity of an alkylation, due to the steric interactions induced by the amine substituents. Try to predict where a substituent on the 6-position would go (axial or equatorial) and where an incoming alkylating agent will react (syn or anti).
Back to CH630 Home Page
Last updated: 09/25/03
Comments to K. Gable