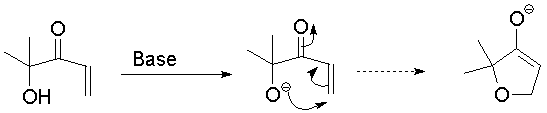
Examining the interaction between oxygen and carbon, we see that the overlap between the electrons on oxygen and the LUMO of the enone is minimal; oxygen lies along the node of the MO.
Show the LUMO
Clear orbitals
This ring closure is unsuccessful.
The general observation that certain ring sizes are easier to make via a cyclization reflects both the enthalpic stability of the transition state and the entropic probability that the nucleophilic and electrophilic sites will encounter each other with the proper geometric orientation. A molecular orbital argument is that there must be sufficient spatial interaction between the HOMO (or the specific MO containing the nucleophilic electrons) and the LUMO (or some other low-energy acceptor orbital arising from the breaking bond). These arguments have been systematized and summarized by Baldwin and coworkers (J. Chem. Soc., Chem. Commun., 1976, 734).
Compare two 5-member ring closures.
The first is a 5-endo-trig closure:
 Examining the interaction between oxygen and carbon, we see that the overlap between the electrons on oxygen and the LUMO of the enone is minimal; oxygen lies along the node of the MO. |
|
|
Show the oxygen lone pair Show the LUMO Clear orbitals This ring closure is unsuccessful. |
|
A successful ring closure is a 5-exo-tet closure:
 |
||
| Malonate5.pdb |
Show the interacting atomic orbitals Show the actual HOMO Show the C-Br antibonding MO Clear orbitals This ring closure works. |
|
The ease with which the HOMO can overlap the LUMO assists the development of a new C-C bond and speeds the reaction.
The summary of the rules is listed below (X = unfavorable, ! = favorable).
| Ring size | Endo-Dig | Endo-Trig |
| 3 | ! | X |
| 4 | ! | X |
| 5 | ! | X |
| 6 | ! | ! |
| 7 | ! | ! |
| Ring size | Exo-Dig | Exo-Trig | Exo-Tet |
| 3 | X | ! | ! |
| 4 | X | ! | ! |
| 5 | ! | ! | ! |
| 6 | ! | ! | ! |
| 7 | ! | ! | ! |
Back to CH 630 Home page
Last updated: 09/29/2003