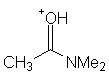
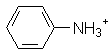
Acidity of Representative Organic and Inorganic Acids
For some particularly thorough comparisons of pKa values, consult:
Note that the pKa value will be different in different solvents; consult one of the above texts for a complete description of how the pKa value is determined. Tables below are intended to give you a comparative list of Bronsted acids and their relative acidity. The quantitative application of this data requires knowing the pKa for all acids in the solvent of interest.
Some representative values.
Inorganic and sulfonic acids.
| Acid | Conjugate Base | pKa |
| HCl | Cl- | -8 |
| CF3SO3H | CF3SO3- | -5.9 |
| PhSO3H | PhSO3- | -2.8 |
| H2SO4 | HSO4- | -2.8 |
| H3O+ | H2O | -1.7 |
| H3PO4 | H2PO4- | 2.1 |
| HF | F- | 3.2 |
| H2CO3 | HCO3- | 3.7 |
| H2S | HS- | 7.0 |
| NH4+ | NH3 | 9.2 |
| HCO3- | CO3-2 | 10.2 |
| HOOH | HOO- | 11.6 |
| H2O | HO- | 15.74 |
Protonated organic molecules
| Acid | Conjugate Base | pKa |
| Me2SH+ | Me2S | -6.99 |
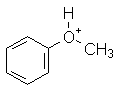
 |
PhOCH3 | -6.5 |
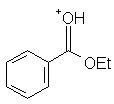
 |
Ethyl benzoate | -6.2 |
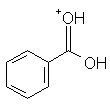
 |
Benzoic acid | -4.7 |
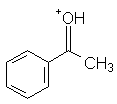
 |
Acetophenone | -4.3 |
 |
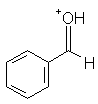
Benzaldehyde | -3.9 |
| Me2OH+ | Me2O | -3.8 |
| (CH3)2C=OH+ | Acetone | -2.85 |
| CH3OH2+ | Methanol | -2.5 |
 |
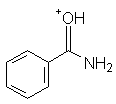
Benzamide | -1.74 |
| (CH3)2S=OH+ | DMSO | -1.5 |
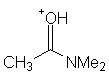 |
N, N-dimethylacetamide | 0.1 |
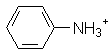 |
Aniline | 4.6 |
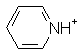
 |
Pyridine | 5.2 |
| Cyclohexylamine | 10.7 | |
| Et3NH+ | Triethylamine | 10.8 |
| Et2NH+ | Diethylamine | 11.0 |
Carboxylic Acids
in water unless otherwise noted
| Acid | Conjugate Base | pKa |
| CF3CO2H | CF3CO2- | -0.6 |
| CCl3CO2H | CCl3CO2- | -0.5 |
| HO2CCO2H | HO2CCO2- | 1.25 |
| Cl2CHCO2H | Cl2CHCO2- | 1.35 |
| FCH2CO2H | FCH2CO2- | 2.6 |
| ClCH2CO2H | ClCH2CO2- | 2.86 |
| HCO2H | HCO2- | 3.75 |
| PhCO2H | PhCO2- | 4.2 |
| CH3CO2H | CH3CO2- | 4.76 |
| PhCO2H (DMSO) | PhCO2- | 11.0 |
| CH3CO2H (DMSO) | CH3CO2- | 12.3 |
Alcohols and amines
| Acid | Conjugate Base | pKa |
| p-Nitrophenol | p-Nitrophenoxide | 7.2 |
| Phenol | Phenoxide | 10.0 |
| EtSH | EtS- | 10.6 |
| CF3CH2OH | CF3CH2O- | 12.4 |
| Imidazole |
14.5 | |
| CH3OH | CH3O- | 15.5 |
| CH3CH2OH | CH3CH2O- | 15.9 |
| (CH3)2CHOH | (CH3)2CHO- | 17.1 |
| (CH3)3COH | (CH3)3CO- | 17.2 |
| Ph2NH (DMSO/H2O) | Ph2N- | 22.4 |
| iPr2NH (THF) | iPr2N- | 35.7 |
| NH3 | NH2- | 41 |
"Activated" C-H bonds
| Acid | Conjugate Base | Solvent | pKa |
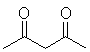 |
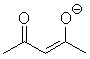 |
H2O | 9.0 |
| DMSO | 13.4 | ||
| CH3NO2 | -CH2NO2 | H2O | 10.0 |
| DMSO | 17.2 | ||
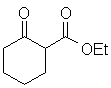 |
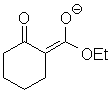 |
H2O | 10.9 |
| NC-CH2-CO2Et | NC-CH--CO2Et | DMSO | 12.8 |
| CH3CO-CH2-CO2Et | CH3CO-CH--CO2Et | DMSO | 14.2 |
| H2O | 11 | ||
| CH3SO2-CH2-SO2CH3 | CH3SO2-CH--SO2CH3 | H2O | 12.5 |
| EtO2C-CH2-CO2Et | EtO2C-CH--CO2Et | H2O | 13 |
| CH3CHO | -CH2CHO | H2O | 16.5 |
| Acetone | -CH2COCH3 | H2O | 19.2 |
| DMSO | 26.5 | ||
| CH3SO2Ph | -CH2SO2Ph | DMSO | 29 |
| CH3CO2Et | -CH2CO2Et | DMSO | 30.5 |
| CH3CN | -CH2CN | DMSO | 31.2 |
Hydrocarbons
All are in DMSO unless otherwise noted.
| Acid | Conjugate Base | pKa |
| 18.1 | ||
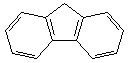 |
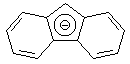 |
22.6 |
| HCCH | HCC- | 25 |
| Ph3CH | Ph3C- | 30.6 |
| Ph2CH2 | Ph2CH- | 32.3 |
| PhCH3 | PhCH2- | 43 |
| Benzene | Ph- | 43 |
| CH2=CH2 | CH2=CH- | 44 |
| CH3CH=CH2 | -CH2-CH=CH2 | 48 |
| CH3-CH3 | -CH2-CH3 | 50 |
| CH4 | -CH3 | 58-60 |
Back to CH 630 Home page
Last updated: 09/21/00