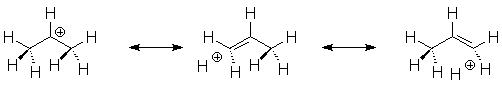
Hyperconjugation
One of the ways alkyl groups stabilize carbocations is via hyperconjugation. We can use resonance structures to illustrate this in the 2-propyl cation:

Alternatively, MO theory can show us the effects of this partial electron donation. In the picture below, the methyl groups are sequentially rotated (by 22.5°) through a 90° dihedral rotation. Note how the contribution of the methyl hydrogens changes as the C-H bonds align (or twist out of alignment) with the empty p orbital at C-2:
Energies are as follows (AM1; solvation with water; kcal/mol): the LUMOs are displayed showing the degree of interaction with the various C-H orbitals.
| Erel = 0 | Erel = +0.5 kcal/mol | Erel = +1.0 kcal/mol |
Back to CH630 Home Page