Answers to Problem Set 6.
7.2
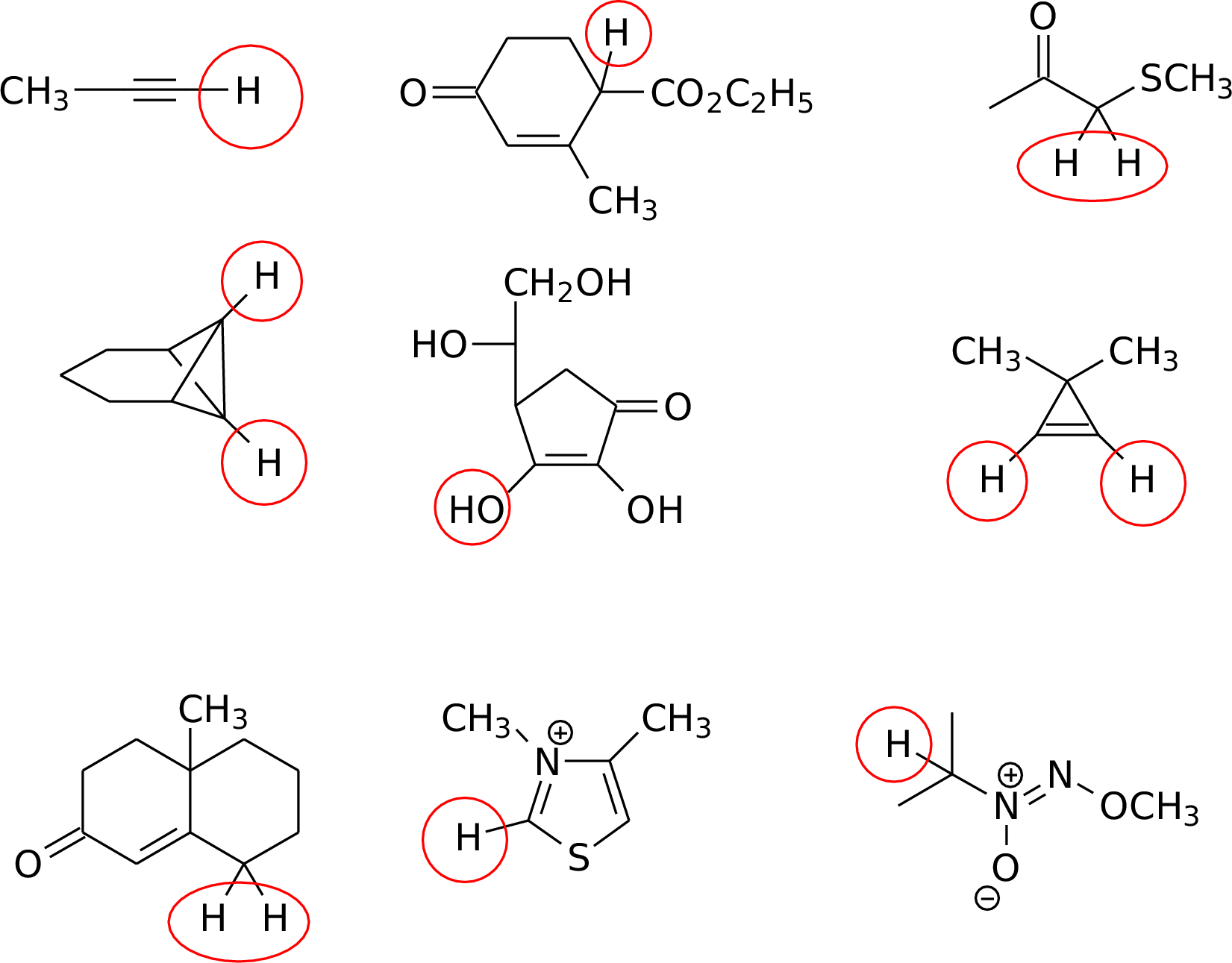
7.4
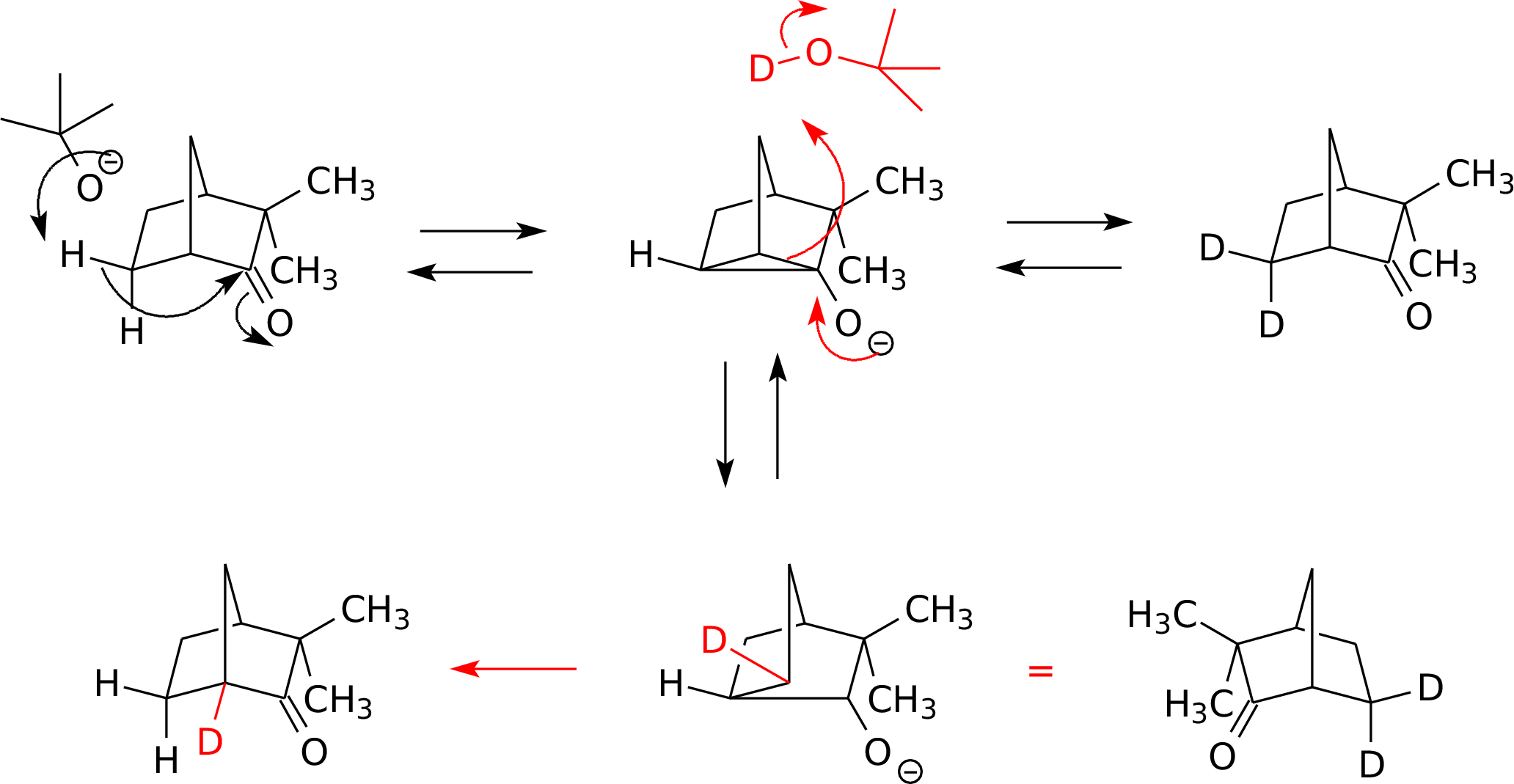
a. Deprotonation (and the resonance delocalization necessary to
allow this) requires the enolate pi bond be placed in an
already-strained bicyclic ring. The [2.2.1] system can
accommodate this more readily than the [2.1.1] system.
b.
7.8.This should (and does) correlate best with sigma, because the
structural relationship of the developing charge to the substituents on
the ring is identical to that in benzoate ions.
7.9. a. Attack of H-; collapse of the
tetrahedral intermediate with expulsion of Ph3C- (a
highly resonance-stabilized carbanion). The benzaldehyde is
further reduced.
b. Pyridine derotonates OH; the hemiacetal opens. Repeat to
form two molecules pf PhCH(OH)CHO. Enolization allows
isomerization to PhC(O)CH2OH.
c. Deprotonation of OH, then ring opening to the benzylic
carbanion.
d. Deprotonation of the hemiacetal; ring opening of the two acetal rings to form a
dialdehyde. This can enolize to racemize the final
stereocenter. Note carefully that all stereocenters must invert to
racemize!
e. Deprotonation of the vinylogous position; protonation to form
the nonconjugated enone; deprotonation of the opposite side of the
ketone; reprotonation to form the conjugated enone.
7.17.This is the "reverse sense" of the anomeric effect. There is
electron-electron destabilization when the lone pair on carbon is
placed in an axial orientation. (The fully correct statement is
that placing the C-H axial is preferred because of the interaction of
the C-H antibonding MO with the lone pairs on sulfur.)
7.21
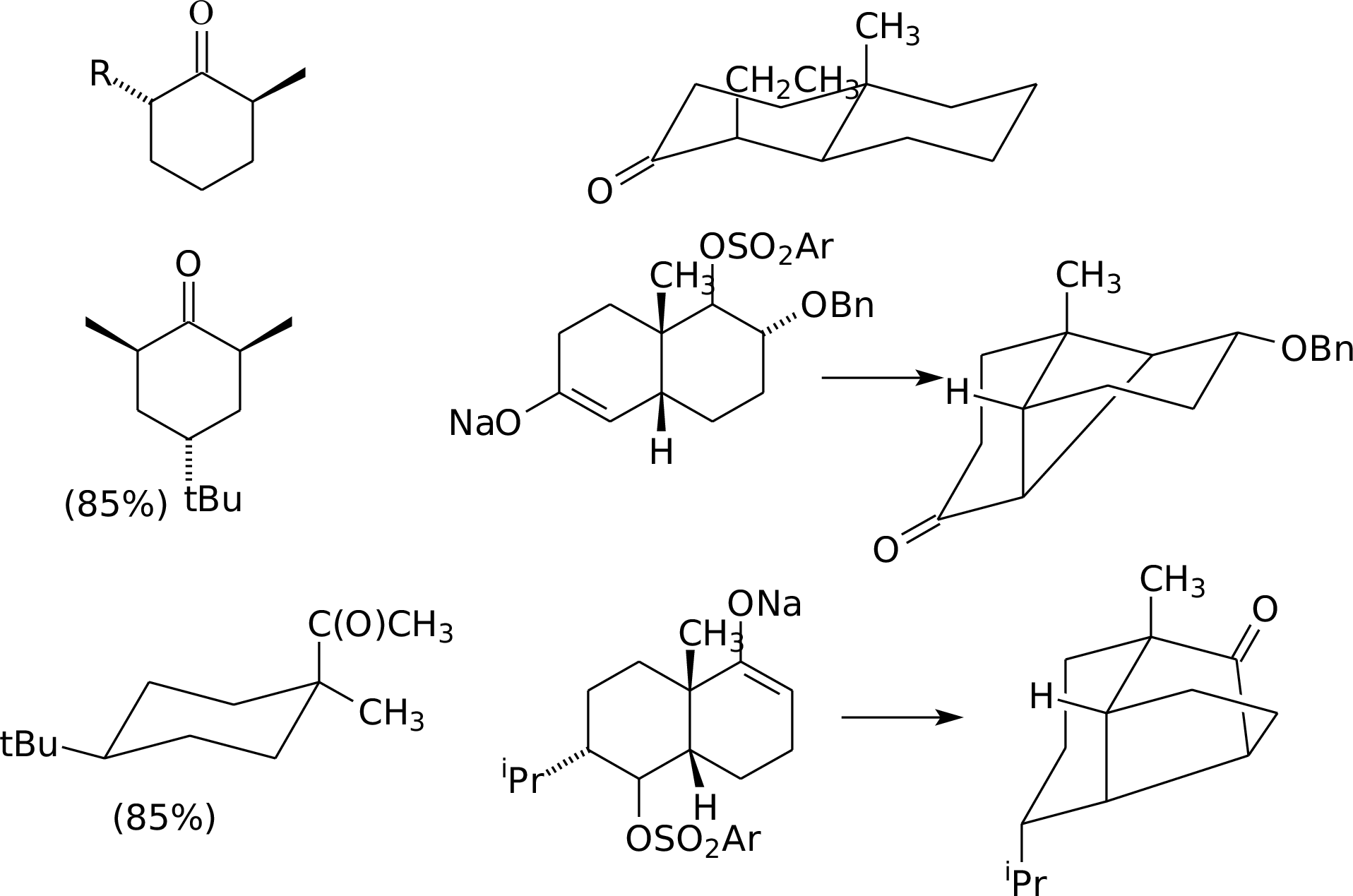
Section B.
The data tells you the following:
1. Each Li is bound to 2
nitrogens.
2. The triethylamine can coordinate to Li, and addition of this
electron density makes the amide nitrogen a kinetically better base.
3. THF is a better coordinator, but not as good an electron
donor. Therefore, a change in aggregation is unlikely to be the
principle effect.
4. This is a normal, primary KIE: the proton is transferred
in the RDS.
See http://dx.doi.org/10.1021/ja021284l
(on-campus access
required) for a diagram of the proposed TS.
Back to Homework
page
Back to CH 630 Home
page
Last updated:
12/01/2003



