The Hammond Postulate
The Hammond postulate suggests (as an unprovable assertion) that species that are
sequential on the reaction coordinate and similar in energy are therefore similar in
structure. Conversely, changes in structure that stabilize or destabilize reactive
intermediates will stabilize or destabilize transition states leading to them. This
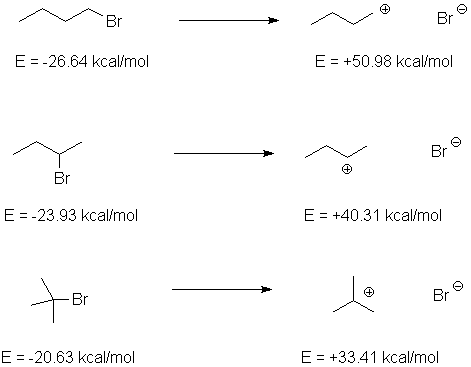
can be illustrated by ionization of different bromobutanes and measured by the rate of
reaction with water at 50°C. Compare this to the energies of the reactants, and the
ionized intermediates, as calculated at the AM1 level (with Cramer-Truhlar solvent
correction for water).

We can compare the relative energies of the cations with the reaction rate, which
reflects the energy of the transition state relative to the ground state:
| Cation Structure | Erel of ions, kcal/mol (AM1) | krel, 50°C, for ionization |
| 1-Bromobutane
Show the ESP map |
77.62 | 1.0 (for SN2; SN1 is slower but not observed) |
| 2-Bromobutane
Show the ESP map |
64.33 | 11.6 |
| t-Butyl Bromide Show the ESP map |
54.01 | 1.2 x 106 |
The nature of the alkyl stabilization derives from the electron-donating ability of the alkyl group; look at the electrostatic potential maps for each ion (mapped on a common scale).
Blue represents positive electrostatic potential, and red represents negative potential.
Back to CH630 Home Page
Last updated: 09/21/00
Comments to K. Gable