Carbocation stability has the predominant impact on SN1 reactivity. Compare structures for calculated transition states for the following 4 SN1 reactions, along with their relative energies. (Note that capture of the nucleophile by the carbocation will be a rapid second step in each case.)
|
Page Controls: Black background White background | Spacefilling model Wireframe Ball & Stick |
|
| Reactants |
TS structure |
Carbocation structure and energy |
| PhCH2Cl |
Benzylchloride_TS.pdb C-Cl = 3.599 Erel=+18.1 kcal/mol (+75.7 kJ/mol) | PhCH2+ Erel = +16.1 kcal/mol |
| tBu-Cl
|
chloro_i_butane_TS.pdb C-Cl = 3.695 Erel=+15.7 kcal/mol (+65.8 kJ/mol) |
Me3C+ Erel = +9.4 kcal/mol |
| iPr-Cl
|
chloropropane_TS.pdb C-Cl = 3.53 Erel=+27.4 kcal/mol (+114.8 kJ/mol) |
Me2CH+ Erel = +20.6 kcal/mol |
| CH3CH2-Cl
|
chloroethane_TS.pdb C-Cl = 4.40 Erel=+35.9 kcal/mol (+150.4 kJ/mol) |
(CH2CH2)H+ Erel = +29.7 kcal/mol |
The results fit closely with observation: tertiary, conjugated, and other stabilized carbocations are formed rapidly, while secondary (non-resonance stabilized) carbocations form slowly and primary not at all (except under very rare, forcing conditions where other chemistry starts to happen).
For the "primary carbocation" formation, note that one of the hydrogens bridges! This is because of the intrinsic instability of the primary carbocation--primary substrates do not undergo SN1 substitution!!
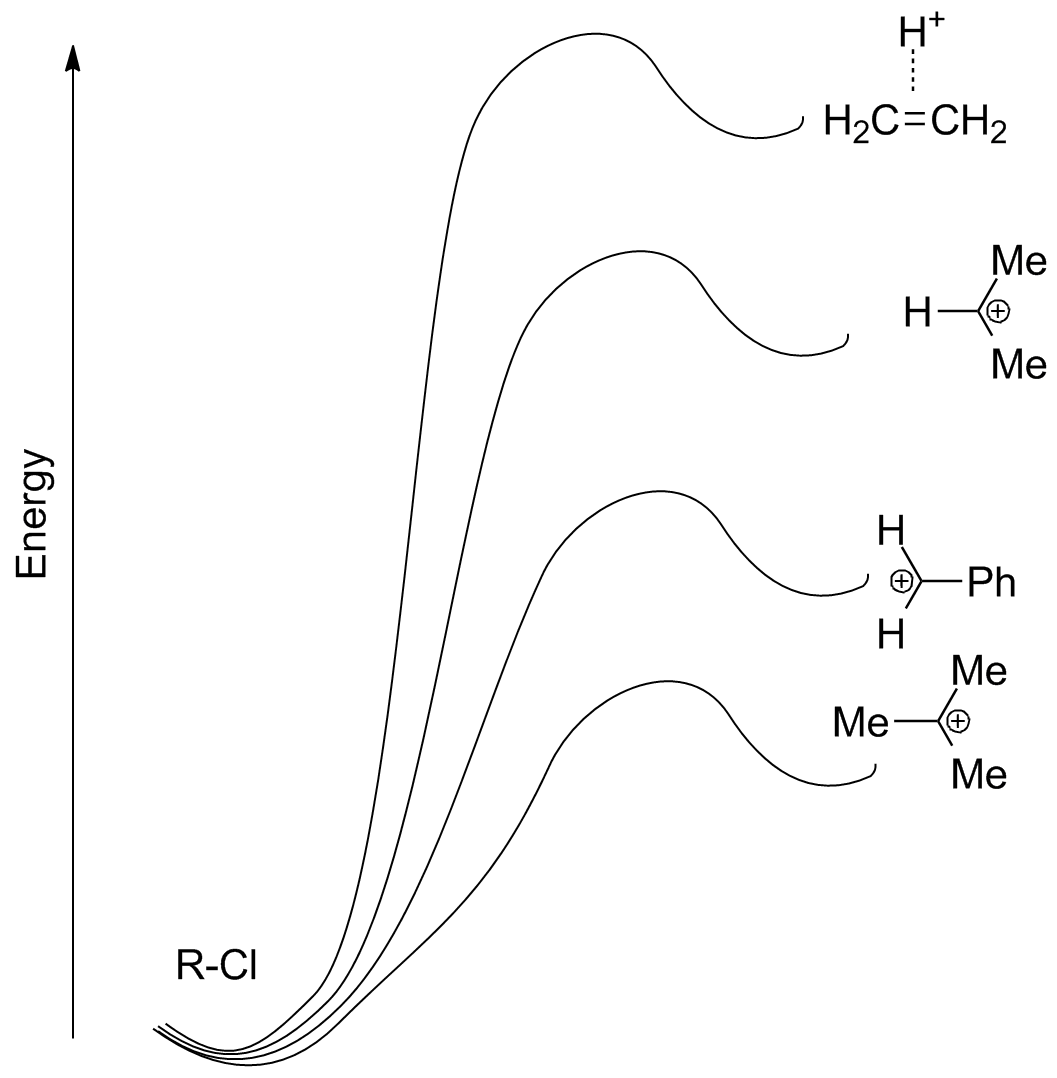
A pictorial representation shows how stabilization of the carbocation lowers the energy barrier (and therefore increases the SN1 reaction rate)