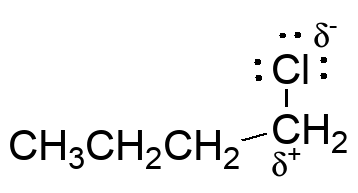
The chlorine is a Lewis base and will react with Lewis acids; this enhances the electron deficiency at carbon by drawing away even more electron density.
Probably the most difficult example to see, but the polarization of the C-Cl bond creates partial electron def
iciency at carbon.
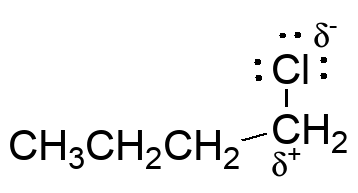
The chlorine is a Lewis base and will react with Lewis acids; this enhances the electron deficiency at carbon
by drawing away even more electron density.