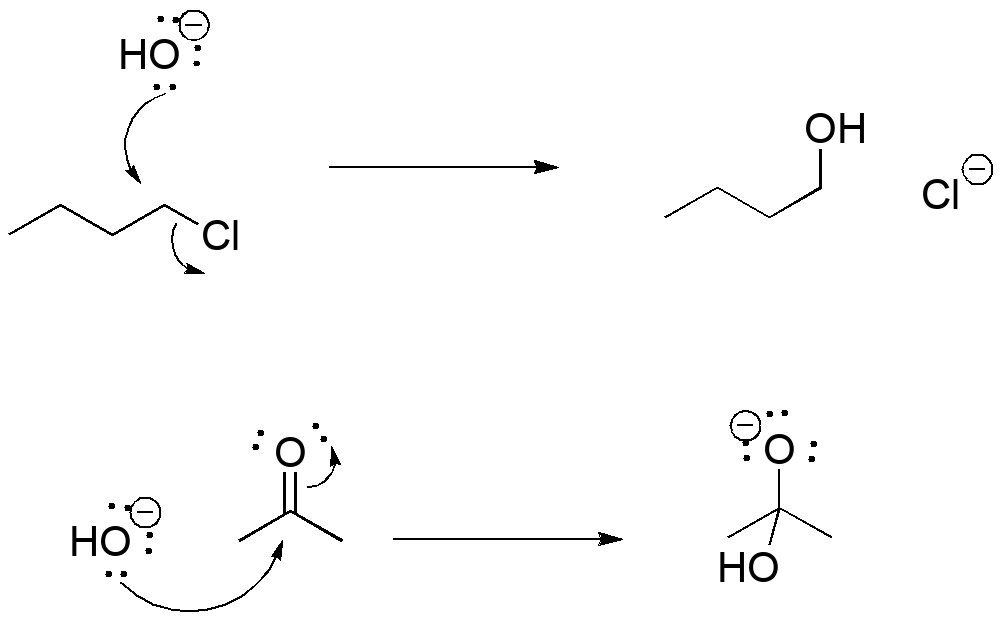
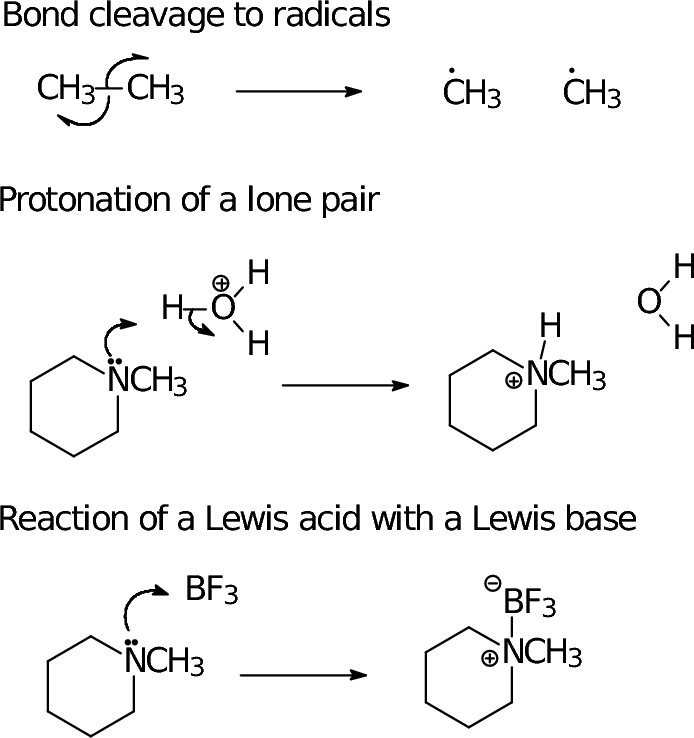
In organic chemistry, we use a formalism called "electron pushing" to describe bond reorganization. We've started to use this as we draw different resonance forms of an unsaturated molecule as a bookkeeping tool:
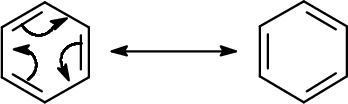
Now it's time to understand this in depth.
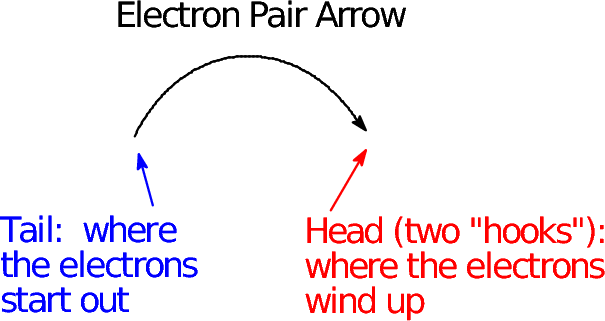
- The tail of the arrow is placed on the electron(s) that move. Often this is a bond line; it can also be an explicit lone pair, an unpaired electron, or (understanding that a negative charge implies a lone pair) a negative charge symbol.
- The head of the arrow points to where the eletron(s) wind up. If we make a new bond, that's between the atoms now bonded; if we break a bond, it's the atom that gains formal possession of the electrons.
- The arrow head tells how many electrons
move: a "regular" arrow head implies an electron pair; a
"fishhook" arrow head implies a single electron.
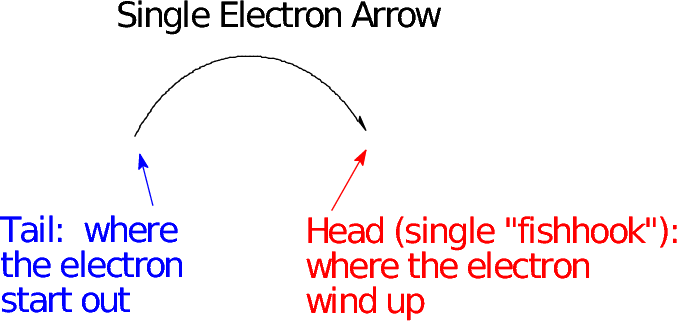
- Just as in writing resonance structures, it is important to have a reaction depicted between proper Lewis structures of both reactant and product.
- As a consequence, when the electron flow (arrow) is toward an atom with a complete octet, that atom must also lose an electron pair (or electron, if it's a "fishhook" arrow) during the reaction--one of the bonds needs to break so the octet rule is not violated.
Two examples of reactions of nucleophiles: