Bromide Ion
A very simple example is any of the halide ions:
monoatomic, with a complete octet (4 electron pairs). High electron density makes Br-
a typical and good Lewis base. The negative charge enhances the behavior.
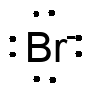
Look at the ESP map to see that the bromide does, in fact, have a negative electrostatic pot
ential (red) and will be attracted to sites of electron deficiency and positive charge.