Look at the ESP map to see that the boron does, in fact, have a positive electrostatic potential (blue) and wi ll be attracted to negatively-charged electrons.
Probably the most common example, and used extensively in the text.
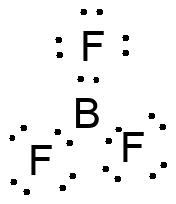
Boron compounds are often trivalent, leaving a site of electron deficiency on B. Interaction with an electr
on pair in another molecule completes the octet at boron. Many neutral boron compounds act as strong Lewis ac
ids.

Look at the ESP map to see that the boron does, in fact, have a positive electrostatic potential (blue) and wi
ll be attracted to negatively-charged electrons.