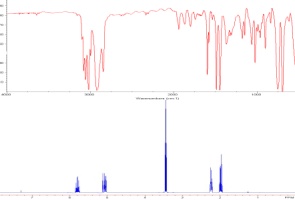
The C=O group is one of the
most easily recognized peaks in an IR spectrum. The change in dipole
moment is significant, making this an intense band, and there are few
other groups that give rise to absorbances in the 1600-1850 cm-1
range. Furthermore, the precise position can be readily correlated with
resonance effects: the higher the C=O bond order, the higher the
frequency. Resonance donation of electron density, conversely, lowers
bond order and decreases the absorbance frequency.
| Ketones: normally 1720 cm-1 |
Cyclohexanone: 1710 cm-1 |
| Aldehydes: Somewhat higher in frequency, 1735 cm-1 |
Cyclohexanecarbaldehyde:
1740 cm-1 Note also the doublet at 2706 and 2807 cm-1,
which is a characteristic aldehyde C-H feature. |
| Conjugated
aldehydes and ketones: Presence of a conjugated Π bond or an
aromatic ring will lower the frequency by 20-30 cm-1. There
will often be combinations with the C=C stretch. |
Acrolein:
1734, 1710 cm-1
Benzaldehyde:
1711 cm-1
Methyl vinyl
ketone: 1707 cm-1
Acetophenone:
1690 cm-1
|
| Ring size can play an important role for cyclic
ketones; small rings impose strain and increase the net C=O bond order. |
Cyclobutanone:
1810 cm-1
Cyclopentanone:
1774 cm-1
Cyclohexanone:
1710 cm-1
Cycloheptanone:
1722 cm-1 |
| Carboxylic acids & derivatives: highly dependent on
resonance donation versus electronegativity. |
Benzoyl
chloride: 1790 cm-1. Note that this is also lowered by
conjugation; alkyl substituted acyl chlorides will be higher.
Propionic
anhydride: In-phase and out-of-phase combinations at 1830 and 1770
cm-1. This is typical behavior for an anhydride.
Ethyl acetate:
1734 cm-1
2-Ethylhexanoic
acid: 1699 cm-1. Note that the broad O-H band is also a
dead giveaway.
Acetamide:
1684 cm-1 |
|