Like the 1H spectrum, the 13C spectrum is referenced to TMS (defined as 0 ppm). However, the chemical shift range is much broader; generally 0-230 ppm for most organic compounds. Here also, there are general chemical shift ranges for different types of carbons.
0-40 ppm Aliphatic carbons. Sp3 hybridized, and not attached directly to an electronegative element. Electrochemical deshielding does affect the chemical shift, but branching of alkyl chains can also lead to a downfield shift.
40-90 ppm Aliphatic carbons next to electronegative elements such as oxygen. Note that CDCl3 (a common NMR solvent) gives a triplet at 77.0 ppm.
90-115 ppm Alkyne region: sp hybridized carbon. Also, carbons bearing two oxygens (acetals, ketals) occur here, as do nitrile carbons.
115-160 ppm Sp2 region; both double bonds and aromatic rings occur here. Normally aromatic carbons are further downfield, but this cannot be generalized.
160-190 ppm Carbonyl region for carboxylic acids and derivatives (esters, acid chlorides, amides, anhydrides).
190-230 ppm Carbonyl region for ketones and aldehydes.
Note that one important application of 13C NMR is the deduction of the number of symmetry-inequivalent carbons.
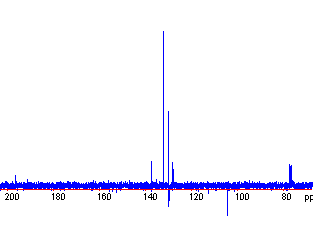
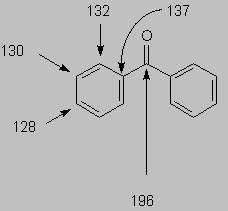
Example: benzophenone exhibits 5 peaks in the 13C spectrum (instead of 13 as suggested by its molecular formula):
 |
 |
There are 4 in the aromatic region, and 1 carbonyl.
Note some other features. There are a set of 3 peaks at 77 ppm; these come from solvent (CDCL3 is the most common NMR solvent; C-D coupling creates a triple peak). Also, there are some noise spikes that dip below the baseline. Finally, the intensity of the signal varies from carbon to carbon. Intensity is not a reliable measurement of the number of carbons, for reasons that are beyond the scope of this course.
Return to NMR page
Return to CH362 Home Page
09/09/02-->
Comments to K. Gable